You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Patients present for tooth extraction for various reasons (eg, caries, periodontal disease).1 The dental literature describes loss of bone volume after dental extractions of up to 50% within 6 months.2-4 The effect of bone loss is magnified when multiple teeth are extracted in the same area.5 Bone resorption will result in a loss of socket width in an apical and lingual direction. Socket collapse can prevent or significantly inhibit the placement of dental implants in ideal, prosthetically driven positions.6 Severe loss of alveolar volume may necessitate ridge augmentation by block grafting and other extensive surgical procedures if dental implants are to be placed for the support of a prosthesis.7
The purpose of this pilot study was to evaluate the clinical effects on alveolar ridge preservation after placement of particulate beta-tricalcium phosphate (β-TCP) graft (Cerasorb®, Curasan AG, www.curasan.de) placed at the time of tooth extraction.8-12 The graft material was mixed with blood from the site, grafted to fill the area to ideal contour, and covered with an occlusive barrier. Histologic evaluation of the regenerated material was performed from a core taken at 6-month reentry, concurrent with implant placement. Unlike other bone replacement graft materials currently used for this type of procedure,13-15 this graft material has been shown to resorb fully and be replaced by vital alveolar bone in 6 to 10 months.16-19 In this study, this technique of graft and barrier placement led to 100% successful implant placement, maintenance of 90% of buccolingual socket width, resorption of the graft material, and formation of vital bone in the sockets.
Materials and Methods
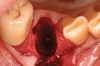
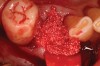
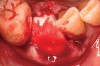
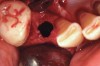
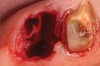
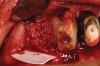
Thirty patients were selected on the basis of need for dental extractions with bone preservation and/or augmentation in the socket before the placement of a dental implant. These patients were cleared medically for oral surgical procedures. Preoperative clinical photographs and periapical radiographs were taken. After administration of local anesthesia, full-thickness labial and lingual/palatal flaps were elevated. Minimal soft-tissue manipulation was performed to allow visualization of the entire alveolar crests and debridement of fenestrations and/or dehiscence defects, if present. Elevation of each tooth or retained roots was performed, using periotomes, luxatomes, proximators, root forceps, and any other instruments and procedures as required to extract the tooth with minimal trauma (Figure 1). After extraction and thorough debridement of the socket by mechanical means, clinical photographs were taken. The sites then were grafted with a resorbable β-TCP of small particle size, 150 µm to 500 µm (Cerasorb) (Figure 2). This material has been used and reported in the literature for almost 30 years as a viable bone graft in orthopedic and other surgical specialties. In this study, the granules of β-TCP were mixed thoroughly with blood from the surgical sites and the sockets filled to ideal contours faciolingually and apicocoronally. The grafts and adjacent 3 mm of alveolar bone then were covered with resorbable collagen barriers (BioMend®, www.zimmerdental.com) (Figure 3). The flaps were repositioned and the areas closed with an appropriate number and type of sutures. No attempts were made to obtain primary closure over the exposed barriers. Postoperative radiographs and clinical photographs were taken. Subsequently, sutures were removed at 1 to 2 weeks.
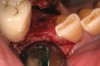
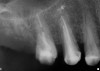
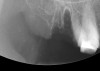
The grafted sites were followed clinically and radiographically throughout the healing period. Healing of the residual crestal defects (if present) also was followed. At approximately 6 months, the patients returned for graft analysis and placement of implants. Clinical photographs and radiographs were taken preoperatively. After administration of local anesthesia and minimal flap elevation, measurements of alveolar ridge width were repeated to compare with the preoperative alveolar dimensions (Figure 4). The coronal portions of the regenerated sites were evaluated for the presence of residual graft material. Preparation of the osteotomies was initiated with a bone trephine to sample the coronal 5 mm to 7 mm of socket healing (Figure 5). The placement of appropriately sized dental implants was performed according to the manufacturer’s recommendations, and the flaps were sutured to facilitate both soft- and hard-tissue healing.
Histologic Preparation and Histomorphometry
Bone cores were harvested from the surgical sites at the time of dental implant placement. The trephines containing the bone were fixed in 10% neutral buffered formalin. On receipt in the Hard Tissue Research Laboratory at the University of Minnesota School of Dentistry, the specimens were dehydrated immediately with a graded series of alcohols for 9 days. After dehydration, the specimens were infiltrated with a light-curing embedding resin (Technovit 7200 VLC, Heraeus Kulzer Technical Division, www.heraeus-kulzer-us.com). After 20 days of infiltration with constant shaking at normal atmospheric pressure, the specimens were embedded and polymerized by 450-nm light, with the temperature of the specimens never exceeding 40°C. The specimens then were prepared with the cutting/grinding method of Donath.20,21 The specimens were cut to a thickness of 150 µm on an EXAKT cutting/grinding system (EXAKT Technologies, Inc, www.exaktusa.com). Next, the slides were polished to a thickness of 45 µm, using the EXAKT microgrinding system followed by alumina polishing paste and stained with Stevenel’s Blue and van Gieson’s picrofuchsin. After histologic preparation, the cores were evaluated morphometrically. All the cores were digitized at the same magnification using an Axiolab microscope (Carl Zeiss MicroImaging Inc, www.zeiss.com) and a Coolpix® 4500 digital camera (Nikon Americas Inc, www.nikonusa.com). Histomorphometric measurements were completed using a combination of Adobe® Photoshop® (Adobe Systems, Inc, www.adobe.com) and the National Institutes of Health’s image program (available to the public at http://rsb.info.nih.gov/nih-image). At least two slides of each core were evaluated. Parameters evaluated were total area of the core, percentage of new bone formation, and percentage of residual graft material.
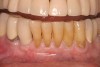
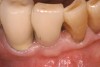
At the appropriate time after implant placement, restorative procedures were performed. The implants were restored with fixed ceramometal restorations to return the patients to ideal form and function (Figure 6 and Figure 7). Alveolar crestal heights were followed radiographically from the times of extraction through placement of the final restorations to assist in determination of stability of the crestal attachment apparatuses.
Results
In the 30 patients who entered this study, 21 sites have received dental implants as of this writing. The healing of all sites grafted at the time of extraction with β-TCP was uneventful. In five of the sites where there was no primary closure over a minimally exposed portion of the membrane, reepithelialization occurred within the first 2 weeks postoperatively.
The sites that were reentered for dental implant placement demonstrated excellent preservation of buccolingual alveolar ridge width. The initial socket widths ranged from 8 mm to 12 mm, with an average of 9.6 mm. Average loss of buccolingual width for the period studied was 12.4%. Though there were a smaller number of sites at which the grafts were separated by particle size, better ridge width maintenance was obtained with the larger (500 µm to 1000 µm) particle sizes as shown by 92% preservation of the preextraction width (Table 1).

Coronally, in a number of cases, some residual graft particles were visible at the time of osteotomy preparation, which did not affect either the surgical procedure or the initial stability of the dental implant. The osteotomies were prepared and implants inserted in the surgical sites according to the manufacturers’ recommendations. Marginal gingival tissues were well supported to enable functional and esthetic restoration of the implants with fixed prostheses approximately 3 months later.
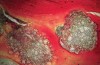
Histologically, in this early period, there was evidence of vital bone ingrowth into the extraction sockets grafted with this pure-phase β-TCP (Figure 8 through Figure 11). New bone formation was present in intimate contact with the surface of the graft particles. Some residual graft particles were present in all specimens investigated. Varying degrees of resorption and remodeling were noted in each of the specimens.
Discussion
Characteristics for ideal bone replacement graft materials include safety, efficacy, and the ability for the graft material to be replaced by vital alveolar bone. The β-TCP graft material used in this study is a purely synthetic material fabricated to exact chemical specifications.22 Porosity (of the β-TCP materials), both macroscopic and microscopic, is designed to maximize blood clot stability during early healing. The material has no organic components and, therefore, no chance of antigenicity or allergic reactions. As the material is synthesized in the laboratory, any possible infectivity is removed. There have been recent concerns regarding possible disease transmission from allografts and xenografts. Using an alloplastic regenerative material prevents this possibility, eliminating the surgeon’s as well as the patient’s apprehension. Chemically, the graft has a similar composition to a salt. Because of the chemical composition and high purity of the material, no cytotoxic compounds are released during breakdown and resorption of this graft material.22,23 Compared with autogenous bone grafting, this synthetic graft has unlimited availability without the increased potential for postoperative morbidity from the harvesting procedure.
In previous papers on dental extraction therapy with or without immediate socket implants, site collapse in a buccolingual dimension was ≥ 50% for a 6-month period. Studies have demonstrated preservation of alveolar dimensions after extraction socket grafting.24-26 These studies showed no treatment of the socket resulted in a collapse of the alveolus by 29% and grafting the socket at the time of extraction resulted in bone loss of only 13%.24 The average loss of alveolar width in the grafted sites in this study was 12%. Numerous papers state that one of the reasons to place an immediate socket implant is to preserve the alveolar ridge width. That statement is not borne out consistently in the conclusions of the authors. One recent paper, in which reentry was performed at the time of dental implant uncovering, offers an alternative conclusion. In that paper, implants were placed after extraction of single-rooted teeth. After averaging the ridge width changes, it was noted that there was a 35.2% loss of bone volume in the buccolingual dimension.25 Similar evidence of socket collapse after placement of immediate socket dental implants questions the use of this technique to preserve alveolar bone volume.26 The results of this current study clearly demonstrate a significantly better maintenance of alveolar ridge dimension than the placement of an immediate socket dental implant with no grafting (Figure 12 through Figure 14).
Other dental research has demonstrated vital bone fill in extraction sockets with other techniques and materials. One combination of graft materials that has been researched widely for more than 10 years is demineralized freeze-dried bone allograft with calcium sulfate.27-29 While the technique has proven effective, human and some animal-derived bone graft products are not able to be used in a number of countries. In certain instances, the placement of a dense PTFE has been shown to improve vital bone formation when placed over an extraction socket or other surgical defects.30-32 However, sufficient proximal and facial or lingual bone is required for support and stabilization of the barrier for the required period.
The material researched in this paper is a pure-phase β-TCP, which disintegrates eventually and will fully convert to vital alveolar bone. Other bone graft materials that are radiopaque at the time of insertion are resorbed minimally, if at all, during this period,33 giving the surgeon no clue as to the amount of biologic healing that is occurring. In contrast, resorbable grafting materials change radiopacity to appear like normal trabeculated bone as they heal. An advantage of β-TCP is that it will break down clinically, histologically, and radiographically (Figure 15 through Figure 17), providing the surgeon with an indication of the appropriate time to place a dental implant in the grafted site. The sites grafted with β-TCP exhibited a decreased amount of bone loss as compared to nongrafted sites. The treated sites in this study lost, on average, only 12% of the buccolingual width of the original socket dimension. The present results validate the use of this β-TCP for preservation of extraction socket dimensions. The alveolar maintenance provided by this material is comparable with ridge preservation, using other materials discussed in the literature.34 One recent socket treatment study by Iasella et al compared no treatment after extraction to grafting the socket with freeze-dried bone allograft and covering the site with a collagen barrier.24 In the Iasella et al study, the sites where no socket preservation therapy was performed lost, on average, 2.6 mm of an initial 9.1-mm ridge width. The graft- and barrier-treated sites lost only 1.2 mm of the initial 9.2-mm ridge width. In this study, the initial alveolar width was 9.4 mm and the sites lost 1.42 mm on average. The slight decrease of 9.3% in buccolingual dimension in this study compared favorably to the loss of 29% in the untreated sites and the 13% loss in the graft and barrier treated sites 6 months after extraction in that study. Another study by the same research group compared two types of graft- and barrier-protection socket therapies.35 All sockets treated by the two different methods of augmentation lost horizontal ridge width (6% for one group and 5% for the other) but significantly less than untreated sockets.
The results of preservation of alveolar volume after extraction by bone grafting are more favorable than the site collapse seen after placement of immediate socket implants. One study showed that even after placement of immediate socket implants, there was a buccolingual collapse of alveolar width of 56% during the 4-month healing period.36 This negates one of the purported advantages of placing immediate socket dental implants to preserve alveolar ridge dimensions. In their study, Schropp et al performed uncovering surgery at the second stage that enabled the investigators to visualize the alveolar crest.37 Although the alveolar defects were filled with autogenous bone at the time of implant placement, minimal healing resulted. A total of 25% of the initial dehiscence defects healed fully, and approximately 50% of the height and/or width defects healed fully at the visual level. Autogenous bone did not satisfy the criteria of visually filling the gap and becoming bone, even at the macroscopic level, leaving no possibility of osseointegration in that portion of the implant. Another research paper demonstrated similar concerns with osseointegration in alveolar ridges augmented with bovine bone mineral. In another study, edentulous portions of the alveolar ridge in dogs were augmented with bovine bone mineral and allowed to heal.38 Implants were placed 3 months later, followed by abutment connection in an additional 3 months. After allowing the soft tissues to heal for 4 months, the animals were sacrificed. Histologic evaluation in this study revealed alveolar defects between the implants and the nonaugmented portion of the ridges. There was minimal-to-no osseous incorporation of the bovine bone mineral into the alveolar bone during the healing period and no evidence of osseointegration at the alveolar crest in the grafted sites. If bone augmentation were performed with a fully resorbable material that was replaced by vital, alveolar bone, there should be more vital bone and bone-to-implant contact at the crest. This could prevent bone and/or soft-tissue loss around a prosthetically loaded implant.
The results of this clinical study validate the use of this β-TCP for preservation of extraction socket dimensions. The alveolar maintenance provided by this material is comparable to ridge preservation, using other previously researched materials and superior to the bone preservation from conventional immediate socket dental implant therapy. In this current study, the loss of buccolingual width averaged 12% of the original alveolar width. For the cases in which larger particle sizes of pure-phase β-TCP were used, the site width was maintained at 92% of the original socket dimensions. The ability to preserve this volume of alveolar bone and soft tissue after tooth extraction enables the surgeon to place dental implants in these sites ideally. In addition the complete resorption of β-TCP over time, as shown in the histologic specimen analyzed in this study and in dental, orthopedic, and veterinary research, has many benefits.39,40 In one recent study, a fully resorbable β-TCP mixed with bone marrow-aspirated stem cells proved superior to iliac crest grafting for bone formation in an area that normally does not form bone.41 A current published minipig study showed two outcomes essential for implant dentistry.42 Critical-size defects were made in the tibia of minipigs and filled with β-TCP and implants stabilized in the sites. After healing for 5 months, the β-TCP sites showed no histologic presence of inflammation and 70% resorption of the graft materials with vital bone replacement. A later period histologic specimen showed osseointegration with 95% replacement of the graft with vital bone. Use of a resorbable bone-replacement graft material eliminates the possibility of delayed alveolar socket healing, prevents residual graft particles from interfering with osseointegration, and allows complete fill of the socket (or other treated site) with vital alveolar bone.
Conclusions
Clinically, alveolar ridge width was maintained to a very high degree after extraction socket grafting with this β-TCP. As opposed to previous investigations that had shown the loss of 40% to 60% of the buccolingual width of the extraction socket for cases in which no grafting material was used, this study demonstrated almost 90% of the ridge width was maintained on average. Further investigations will be conducted to determine the timeframe for resorption and vital bone ingrowth into the treated sockets. As various particle shapes and sizes may have different resorption characteristics, clinical and histologic evaluation will be performed on these materials, as well. An ideal grafting material for this indication should maintain alveolar socket width and have the ability to be resorbed and replaced by new bone formation. It is clear that the material investigated in this study meets these criteria. The present clinical study validates the use of β-TCP as a useful bone replacement graft at the time of tooth extraction. Clinical measurements showed preservation of alveolar width, and histologic analysis demonstrated both resorption of the graft material and conversion to vital alveolar bone. These characteristics make this graft material ideal for use after tooth extraction in conventional and implant dentistry.
Disclosure
Support for the histologic evaluation, analyses, and clinical materials was provided by Curasan AG. Dr. Mazor and Dr. Horowitz have received honorariums from Curasan AG.
References
1. Al-Shammari KF, Al-Ansari JM, Al-Melh MA, et al. Reasons for tooth extraction in Kuwait. Med Princ Pract. 2006;15(6):417-422.
2. Schoop L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
3. Lam RV. Contour changes of the alveolar processes following extraction. J Prosthet Dent. 1960;10:25-32.
4. Pietrokovski J, Starinsky R, Arensburg B, et al. Morphologic characteristics of bony edentulous jaws. J Prosthodont. 2007;16(2):141-147.
5. Pietrokovski J. The bony residual ridge in man. J Prosthet Dent. 1975;34(4):456-462.
6. Mecall RA, Rosenfeld AL. Influence of residual ridge resorption patterns on implant fixture placement and tooth position. 1. Int J Periodontics Restorative Dent. 1991;11(1):8-23.
7. Nevins M, Camelo M, De Paoli S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19-29.
8. Ferraro JW. Experimental evaluation of ceramic calcium phosphate as a substitute for bone grafts. Plast Reconstr Surg. 1979;63(5):634-640.
9. Köster K, Heide H. Bioaktive Kalziumphosphatkeramik für den Knochen und Zahnersatz. Biotechnische Umschau. 1978;2:226-222.
10. McAndrew MP, Gorman PW, Lange TA. Tricalcium phosphate as a bone graft substitute in trauma: preliminary report. J Orthop Trauma. 1988;2(4):333-339.
11. Foitzik C, Staus H. Le Fort I osteotomy in atrophied maxilla and bone regeneration with pure-phase b-tricalcium phosphate and PRP. Implant Dent. 2003;12(2):132-139.
12. Szabó G, Huys L, Coulthard P, et al. A prospective multicenter randomized clinical trial of autogenous bone versus β-tricalcium phosphate graft alone for bilateral sinus elevation: histologic and histomorphometric evaluation. Int J Oral Maxillofac Implants. 2005;20(3):371-381.
13. Bhaskar SN, Brady JM, Getter L, et al. Biodegradable ceramic implants in bone. Electron and light microscopic analysis. Oral Surg Oral Med Oral Pathol. 1971;32(2):336-346.
14. Cutright DE, Bhaskar SN, Brady JM, et al. Reaction of bone to tricalcium phosphate ceramic pellets. Oral Surg Oral Med Oral Pathol. 1972;33(5):850-656.
15. Artzi Z, Givol N, Rohrer MD, et al. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 2: morphometric analysis. J Periodontol. 2003;74(8):1153-1160.
16. Artzi Z, Weinreb M, Givol N et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and β-tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004;19(3):357-368.
17. Artzi Z, Givol N, Rohrer MD, et al. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 1: description of a dog model and histological observations. J Periodontol. 2003;74(8):1143-1152.
18. Plenk H Jr, Lederer J. Histomorphologie der knochenregeneration nach sinusbodenaugmentation mit zwei formen eines TCP-granulates—ein fallbericht. Z Oral Implant. 2005;1:32-38.
19. Zerbo IR, Zijderveld SA, de Boer A, et al. Histomorphometry of human sinus floor augmentation using a porous β-tricalcium phosphate: a prospective study. Clin Oral Implants Res. 2004;15(6):724-732.
20. Rohrer MD, Schubert CC. The cutting-grinding technique for histologic preparation of undecalcified bone and bone-anchored implants. Improvement in instrumentation and procedures. Oral Surg Oral Med Oral Pathol. 1992;74(1):73-78.
21. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with the attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11(4):318-326.
22. Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25(6):987-994.
23. International Center of Diffraction Data (ICDD), Pennsylvania, USA. Powder Diffraction File PDF 2, Set 55, PDF #55-898 (2005).
24. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
25. Covani U, Cornelini R, Barone A. Bucco-lingual bone remodeling around implants placed into immediate extraction sockets: a case series. J Periodontol. 2003;74(2):268-273.
26. Araújo MG, Wennström JL, Lindhe J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin Oral Implants Res. 2004;17(6):606-614.
27. Sottosanti J, Anson D. Using calcium sulfate as a graft enhancer and membrane barrier. Dent Implantol Update. 2003;14(1):1-8.
28. Sottosanti JS. Calcium sulfate: a valuable addition to the implant/bone regeneration complex. Dent Implantol Update. 1997;8(4):25-29.
29. Anson D. Maxillary anterior esthetic extractions with delayed single-stage implant placement. Compend Contin Educ Dent. 2002;23(9):829-838.
30. Crump B, Rivera-Hidalgo F, Harrison JW, et al. Influence of three membrane types on healing of skull lesions. OOOOE 1996;82(4),365-374.
32. Horowitz RA. Extraction environment enhancement: critical evaluation of early socket healing in long-term barrier-protected extraction sockets [published erratum appears in Compend Contin Educ Dent. 2005;26(11):765]. Compend Contin Educ Dent. 2005;26(10):703-714.
33. Taylor JC, Cuff SE, Leger JP, et al. In vitro osteoclast resorption of bone substitute biomaterials used for implant site augmentation: a pilot study. Int J Oral Maxillofac Implants. 2002;17(3):321-330.
34. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
35. Calixto RF, Teófilo JM, Brentegani LG, et al. Grafting of tooth extraction socket with inorganic bovine bone or bioactive glass particles: comparative histometric study in rats. Implant Dent. 2007;16(3):260-269.
36. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820-828.
37. Schropp L, Kostopoulos L, Wenzel A. Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: a prospective clinical study. Int J Oral Maxillofac Implants. 2003;18(2):189-199.
38. Carmagnola D, Berglundh T, Araújo M, et al. Bone healing around implants placed in a jaw defect augmented with Bio-Oss: an experimental study in dogs. J Clin Periodontol. 2000;27(11):799-805.
39. Franch J, Diaz-Bertrana C, Lafuente P, et al. µ-tricalcium phosphate as a synthetic cancellous bone graft in veterinary orthopaedics: a retrospective study of 13 clinical cases. Vet Comp Orthop Traumatol. 2006;19(4):196-204.
40. Sheats RD, Strauss RA, Rubenstein LK. Effect of a resorbable bone graft material on orthodontic tooth movement through surgical defects in the cat mandible. J Oral Maxillofac Surg. 1991;49(12):1299-1304.
41. Eniwumide JO, Yuan H, Cartmell SH, et al. Ectopic bone formation in bone marrow stem cell seeded calcium phosphate scaffolds as compared to autograft and (cell seeded) allograft. Eur Cell Mater. 2007;14:30-39.
42. Merten HA, Wiltfang J, Grohmann U, et al. Intraindividual comparative animal study of a- and b-tricalcium phosphate degradation in conjunction with simultaneous insertion of dental implants. J Craniofac Surg. 2001;12(1):59-68.
About the Authors
Robert A. Horowitz, DDS, Clinical Assistant Professor, Ashman Departments of Periodontology and Implant Dentistry and Department of Oral Surgery, New York University College of Dentistry, New York, New York; Private Practice in Periodontics and Implant Dentistry, Scarsdale, New York
Ziv Mazor, DMD, Private Practice in Periodontics and Implant Dentistry, Ra’ananah, Israel
Robert J. Miller, DDS, Chairman, Department of Oral Implantology, Atlantic Coast Dental Research Clinic, Palm Beach, Florida; Private Practice, Delray Beach, Florida
Jack Krauser, DMD, Private Practice in Periodontics and Implant Dentistry, Boca Raton, Florida
Hari S. Prasad, BS, MDT, Senior Researcher, Department of Hard Tissue Research, Hard Tissue Research Laboratory, University of Minnesota, School of Dentistry, Minneapolis, Minnesota
Michael D. Rohrer, DDS, MS, Professor and Director, Division of Oral and Maxillofacial Pathology and Director, Hard Tissue Research Laboratory, University of Minnesota, School of Dentistry, Minneapolis, Minnesota