You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The pursuit of prosthodontic precision continues to drive the emergence of increasingly digital protocols for surgical implant placement. Indeed, as the accuracy of surgical implant placement has been shown to greatly influence prosthetic results, the precision afforded by a range of digitally based guidance technologies has been credited with enabling restoratively optimized dental implant procedures.1-4 Stereolithographic static guides are perhaps the prevalent form of surgical guidance for implants.5 Their clinical use, however, can be limited by both manufacturing and positioning errors, as well as by fracture and fabrication time, thus potentially increasing time to treat a patient. Moreover, static guides do not permit real-time changes to the treatment plan in response to surgical conditions while still providing guidance to the clinician.6-10
Unlike the fixed protocols dictated by static stereolithographic resin or laser-sintered metal surgical guides, digital capabilities have emerged that allow dynamic intraoperative adjustments.11-13 The first available dynamic systems provided "navigation," characterized by real-time visual feedback. With navigation, the operator manually matches the current position of a drill intraorally with the plan model derived from cone-beam computed tomography (CBCT) scans of the patient, and this is viewed on a monitor.13 Navigation systems provide information on drill deviation with respect to position or depth; however, unlike with static guides, there is no physical prevention against excursions from the prescribed treatment plan. Therefore, navigation may still be considered an augmented "freehand" approach that is dependent ultimately on the fine motor skill of the operator.12,13
A new class of surgical dental technology, robotic-assisted dental surgery (RADS), offers intriguing novel functionalities. One of these is the concept that, in addition to providing the auditory and visual inputs of navigation, RADS is capable of providing physical guidance through haptic feedback. Robotic haptics function by providing directional and proportional guidance forces and constraining instrumentation trajectory in accordance with the prescribed surgical plan.
Historic Perspective: Robotic Assistance in Medical Surgery
Digital surgery in the hospital setting has a long history, beginning with diagnostic imaging innovations that enabled the development of minimally invasive surgical techniques, including endoscopic, laparoscopic, and arthroscopic surgery,14 and osteotomy surgical guides for orthopedic procedures. Digital technology also facilitated intraoperative navigation, and eventually robotic-assisted surgery emerged.15 Robotic surgery gathered significant momentum in 2000 with the Food and Drug Administration clearance of the da Vinci® robotic system (Intuitive Surgical, davincisurgery.com) in the United States.16 As of today, more than 5,000 surgical robots are in use at hospitals around the world.16 Millions of robotic-assisted surgeries have been performed, across the fields of urology, gynecology, general surgery, orthopedics, neurology, otolaryngology, thoracic surgery, bariatric surgery, and colorectal surgery.15-22
Surgical robotics addressing different clinical needs utilize various approaches to augmenting surgical technique. Robotic systems addressing hard-tissue procedures, such as hip and knee arthroplasty and placement of spine pedicle screws, tend to function as assistive guides, ensuring that surgeon-initiated actions conform to the preoperative digital plan.21,23 In these systems, robotic haptics provide real-time guidance via soft or firm resistance that physically prevents deviation from the plan. Robotic haptic guidance of orthopedic procedures has been reported to enable minimally invasive techniques and has been associated with greater surgical accuracy, minimal tissue trauma, and early functional recovery.17,18,23
Introduction of Robotics Into Dental Surgery
Digital dental implant surgery likewise has developed to improve patient outcomes through the use of enhanced diagnostic imaging, treatment planning software, and assistive surgical systems. Utilization of CBCT has enabled clinical use of static surgical guides and dynamic camera navigation with the goal of attaining accurate, precise, minimally invasive treatment through surgical insights and intraoperative control.
With respect to static surgical guides, CAD/CAM is the means through which the physical guide is generated for use during the drilling sequence and, depending on the manufacturer, to guide the placement of the implants. However, production of surgical guides, whether in-house or by outside laboratories, can delay surgery, and production may need to be repeated if an error or poor fit results. Additionally, guides can break in use, do not permit intraoperative adjustments or allow the operator to assess the soft-tissue type beneath the guide, and may impede irrigation during the osteotomy preparation.6,24 Finally, static guides may be difficult to use in situations when patients cannot open their mouths wide enough to prevent drill head interference on opposing teeth.
Camera navigation systems, also known as dynamic navigation, provide real-time visual information via a visual display of the position of the drill or implant on a monitor with respect to the surgical plan on the patient's CBCT image. In addition, by avoiding fixed (static) guidance, dynamic systems facilitate same-day treatment, avert the risks of broken or inaccurate guides, allow for identification of keratinized tissue at the time of implant osteotomy preparation, and enable irrigation at the surgical site during preparation of the implant osteotomy.
Furthermore, dynamic navigation allows the surgeon to change the plan during the surgery should unexpected intraoperative conditions arise.12,13 However, camera navigation does not physically prevent the surgeon from deviating from the proposed surgical plan or moving beyond the planned depth. Accordingly, the surgeon must watch the monitor rather than the surgical field to ensure the osteotomy and implant placement is proceeding according to plan. Moreover, because camera navigation systems typically use infrared or visible light to track the drill relative to the patient, each aspect of the procedure must remain within the line of sight of the stereoscopic camera, and communication errors may occur during the procedure.12,13
Dynamic surgical guidance arrived in dental implant surgery with the clearance of the first robotic-assisted dental surgery system in 2016.25 As with orthopedic robotic assistance, RADS provides physical haptic guidance that inhibits the surgeon from deviating from the planned implant angulation, location, and depth. Robotic assistance in dental implant surgery potentially offers the promise of combining the advantage of the physical security of static guides with the flexibility and spontaneity of image-based dynamic navigation. A digital robotic-assisted workflow may mitigate the potential for inaccuracies introduced during the production and fitting of a physical guide and allow patients to be treated with same-day guided surgery, while permitting plan modifications during the procedure. At the same time, RADS haptic guidance potentially lets the surgical team focus on the surgical site during the procedure instead of a computer monitor.
In the RADS system used by the authors, the robotic haptic feedback has been designed such that unintentional movement that deviates from the CBCT-based plan is either met with a feeling of resistance or prevented, depending on the stage of the procedure. The system restricts handpiece movement once the handpiece is in the appropriate position in terms of both bodily position and angulation, and prohibits further deeper movement or penetration when the operator drills to depth. The digital nature of RADS haptic guidance allows for same-day guided surgery when indicated and for adjustments to the plan in response to intraoperative decisions. The haptic-based guidance is also designed to allow the surgeon to retain visualization on the anatomy, as opposed to the monitor-based guidance of navigation-only systems.
The following case study outlines a RADS procedure for the prosthetically driven planning and immediate placement of a single-tooth replacement. It includes an analysis of accuracy to the plan.
Case Report
A 64-year-old man presented with a nonrestorable lower left bicuspid (tooth No. 21) whose apex was in proximity to the patient's mental nerve. When assessing whether or not this patient was a candidate for implant placement immediately after extraction, the risk to the mental nerve from the drills tracking down the root socket was a consideration. A RADS approach was recommended to the patient, which would allow for same-day guided surgery to mitigate risk of drill deviation while permitting adjustments to the plan as needed subsequent to the extraction.
The surgical intervention was planned on the same day as the surgery using digital imaging and communication in medicine (DICOM) files from a CBCT scan of the patient. An intraoral splint was mechanically retained on the patient's stable dentition using hard-locking dental material and monitored for proper fixation. A fiducial array was affixed to the intraoral splint during CBCT scanning to allow for eventual registration of the patient location (Figure 1). Using 3D graphics and 2D cross-sections in the RADS planning software (Yomi®-Enabled Surgery, Neocis, Inc., neocis.com), the surgeon virtually optimized the depth and future insertion axis of the 4.1-mm x 10-mm dental implant (SLA® Bone Level, Straumann, straumann.com). The existing tooth was used as a relative guide for restorative-driven planning (Figure 2).
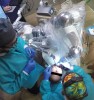
The surgical procedure proceeded with the patient receiving intravenous sedation. Local anesthesia was then administered to the surgical area. Tooth No. 21 was atraumatically extracted. A robotic guidance arm, which held the drill, was then positioned over the patient (Figure 3). A patient tracker arm of the surgery system was attached to the intraoral splint to provide real-time updates on the patient's position throughout the procedure. If the patient moved during the surgical procedure, the RADS system would respond by altering the prescribed surgical cutting angle and position to accommodate the movement.
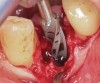
Prior to drilling the osteotomy, a landmark point on the patient scan was verified against direct visualization by placing the drill tip on the chosen anatomical landmark. Surgery proceeded under RADS guidance, in the form of auditory feedback (ie, mode-change and warning beeps) and haptic feedback (ie, resistance to drill motion) as well as visual guidance via a monitor (ie, navigation). To initiate the osteotomy, a 2.3-mm round drill was placed in the robotic guidance arm. As per the implant manufacturer's recommendations, three subsequent drills were used to perform the osteotomy in the prescribed location and angulation (Figure 4 and Figure 5). Implant placement was also achieved with the RADS system (Figure 6 and Figure 7).
The implant was hand-torqued to secure primary stability. A 6-mm x 4-mm healing abutment (Regular CrossFit®, Straumann) was placed and the site grafted with particulate cancellous demineralized bovine xenograft material (Bio-Oss®, Geistlich Biomaterials, dental.geistlich-na.com). The flap was reapproximated around the healing abutment with 3-0 plain chromic gut suture in an interrupted manner, and the splint was removed from the patient's dentition.
A mathematical algorithm was implemented to analyze the deviation between the planned (Figure 2) and actual implant placement (Figure 8). Implant placement versus the plan was evaluated using an automated superimposition of the preoperative plan and the postoperative CBCT plan. In line with methodologies advised in the International Team for Implantology (ITI) consensus reports,5 the algorithm assessed deviation between the planned and actual implant placement in terms of coronal and apical depth, lateral, and global deviations, as well as angular deviation. Results from this RADS case (Table 1) were comparable to those calculated in the ITI consensus meta-analysis of surgical guide accuracy, which reported mean global coronal deviations of 0.9 mm (CI: 95% [0.8-1 mm]), global apical deviations of 1.2 mm (CI: 95% [1.1-1.2 mm]), and angular deviations of 3.3 degrees (CI: 95% [2.1-4.6 degrees]).5
At the time of this writing the patient was healing and awaiting osseointegration of the bone to the implant. A single-tooth, screw-retained prosthesis constructed of porcelain fused to zirconia is planned, and all postoperative signs point to ideal positioning of the implant to support the prosthetic tooth.
Discussion
Since 1999, use of robotic assistance has been rapidly adopted and has assumed an important position across a broad range of medical fields.20-22 Specific to orthopedics, haptics-based robotic guidance has been shown to provide enhanced and augmented surgical precision, tissue preservation, and functional recovery.17,18,23 Only recently has robotic assistance become available in dental implant surgery. This case study is, to the authors' knowledge, the first to outline a robotic-assisted dental surgery procedure and to report on quantitative accuracy for prosthetically driven planning and immediate placement of a single-tooth replacement.
In this immediate implant placement case with minimal residual buccal bone, the surgeon utilized RADS haptics, which enabled steady control of the handpiece to prevent deviation of the osteotomy into the extraction socket. The quantitative results from this case study suggest that the robotic guidance resulted in an accurate osteotomy, which may not be as readily and predictably allowed using freehand protocols. While static guides also provide a high measure of control, their usage may prevent direct visualization of the surgical site, and they cannot be dynamically adjusted if modifications are necessary post-extraction. Furthermore, the RADS system in this case allowed for system verification via a landmark point before preparation of the osteotomy, an asset not available with stereolithographic surgical guides.
Navigation systems also would have allowed for intraoperative changes to the plan but without the haptic guidance of the RADS system to assist in overcoming the tendency of the drill to follow the path of the extraction socket. Additionally, the surgeon was able to retain visualization of the surgical site with RADS, receiving guidance through the sense of touch rather than relying on visual directions on a monitor.
It is important to note that the robotic guidance arm does not move unless the surgeon manually applies a force to it, and motion in guidance mode is constrained within the plan. Otherwise, the robotic guidance arm is able to resist any motion, including incidental bumps or other contact. In this case the surgeon was in control of initiating and pausing drilling and had the ability to modify the plan or switch to freehand operation at any time.
Conclusion
With prosthetically driven implant dentistry now widely considered the ideal approach to offering dental implant therapy, clinicians must execute the virtual plan with as much integrity as possible. The authors' application of a robotic-assisted dental surgery system to this single-tooth immediate implant placement demonstrated accuracy in accordance with published precepts and provided real-time verification of the execution of the plan. This initial experience indicates potential benefits of robotic guidance in comparison with other available digital protocols for prosthetically driven dental implant therapy. It is the authors' intention to follow this RADS system case study with an in-depth multiple case analysis and future prospective studies.
Acknowledgment
The authors thank Robin Grandl, PhD, and Joanne M. Balshi for editorial support.
About the Authors
Sundeep Rawal, DMD
Private Practice, Merritt Island, Melbourne/Suntree, Lake Nona, and Winter Park, Florida; Cofounder, Digital Dentistry Institute
Don E. Tillery, Jr, DMD
Private Practice, Winter Park, Florida
Peter Brewer, DDS
Clinical Specialist, Neocis, Inc., Miami, Florida
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Katsoulis J, Pazera P, Mericske-Stern R. Prosthetically driven, computer-guided implant planning for the edentulous maxilla: a model study. Clin Implant Dent Relat Res. 2009;11(3):238-245.
2. Kosinski T. Proper preparation for prosthetically driven implants: CBCT diagnosing and surgical protocol. Dent Today. 2017;36(6):56-59.
3. Younes F, Cosyn J, De Bruyckere T, et al. A randomized controlled study on the accuracy of free-handed, pilot-drill guided and fully guided implant surgery in partially edentulous patients. J Clin Periodontol. 2018;45(6):721-732.
4. Dano D, Stiteler M, Giordano R. Prosthetically driven computer-guided implant placement and restoration using CEREC: a case report. Compend Contin Educ Dent. 2018;39(5):311-317.
5. Tahmaseb A, Wu V, Wismeijer D, et al. The accuracy of static computer-aided implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(suppl 16):416-435.
6. Sigcho López DA, García I, Da Silva Salomao G, Cruz Laganá D. Potential deviation factors affecting stereolithographic surgical guides: a systematic review. Implant Dent. 2019;28(1):68-73.
7. de Almeida EO, Pellizzer EP, Goiatto MC, et al. Computer-guided surgery in implantology: review of basic concepts. J Craniofac Surg. 2010;21(6):1917-1921.
8. D'Haese J, Ackhurst J, Wismeijer D, et al. Current state of the art of computer-guided implant surgery. Periodontol 2000. 2017;73(1):121-133.
9. Happe A, Fehmer V, Herklotz I, et al. Possibilities and limitations of computer-assisted implant planning and guided surgery in the anterior region. Int J Comput Dent. 2018;21(2):147-162.
10. Kola MZ, Shah AH, Khalil HS, et al. Surgical templates for dental implant positioning; current knowledge and clinical perspectives. Niger J Surg. 2015;21(1):1-5.
11. Block MS, Emery RW, Lank K, Ryan J. Implant placement accuracy using dynamic navigation. Int J Oral Maxillofac Implants. 2017;32(1):92-99.
12. Stefanelli LV, DeGroot BS, Lipton DI, Mandelaris GA. Accuracy of a dynamic dental implant navigation system in a private practice. Int J Oral Maxillofac Implants. 2019;34(1):205-213.
13. Mandelaris GA, Stefanelli LV, DeGroot BS. Dynamic navigation for surgical implant placement: overview of technology, key concepts, and a case report. Compend Contin Educ Dent. 2018;39(9):614-621.
14. Spaner SJ, Warnock GL. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 1997;7(6):369-373.
15. Medical Advisory Secretatiat. Computer-assisted hip and knee arthroplasty. Navigation and active robotic systems: an evidence-based analysis. Ont Health Technol Assess Ser. 2004;4(2):1-39.
16. Smith R. Robotic surgery: the future is already here. LinkedIn Pulse. May 2, 2019. https://www.linkedin.com/pulse/robotic-surgery-future-already-here-roger-smith/. Accessed November 4, 2019.
17. Kayani B, Konan S, Pietrzak JRT, Haddad FS. Iatrogenic bone and soft tissue trauma in robotic-arm assisted total knee arthroplasty compared with conventional jig-based total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplasty. 2018;33(8):2496-2501.
18. Kayani B, Konan S, Tahmassebi J, et al. Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty: a prospective cohort study. Bone Joint J. 2018;100-B(7):930-937.
19. Kayani B, Konan S, Tahmassebi J, et al. An assessment of early functional rehabilitation and hospital discharge in conventional versus robotic-arm assisted unicompartmental knee arthroplasty: a prospective cohort study. Bone Joint J. 2019;101-B(1):24-33.
20. Advincula AP, Wang K. Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol. 2009;16(3):291-301.
21. Kochanski RB, Lombardi JM, Laratta JL, et al. Image-guided navigation and robotics in spine surgery. Neurosurgery. 2019;84(6):1179-1189.
22. Schurr MO, Arezzo A, Buess GF. Robotics and systems technology for advanced endoscopic procedures: experiences in general surgery. Eur J Cardiothorac Surg. 1999;16(suppl 2):S97-S105.
23. Khlopas A, Chughtai M, Hampp EL, et al. Robotic-arm assisted total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int. 2017;30:441-446.
24. D'Haese J, Van De Velde T, Komiyama A, et al. Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: a review of the literature. Clin Implant Dent Relat Res. 2012;14(3):321-335.
25. Food and Drug Administration. K161399 Neocis Guidance System. Silver Spring, MD: Food and Drug Administration; 2016. https://fda.report/PMN/K161399/16/K161399.pdf. Accessed November 4, 2019.