You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Gingival recession has long been a key issue adversely affecting not only dental esthetics but periodontal health as well. The esthetic enhancement of the natural dentition is a significant component of the contemporary dental practice. Adequate zones of keratinized, attached tissue are important for a balanced, harmonious gingival complex as well as long-term periodontal health and maintenance.
Various procedures to correct deficient gingival contours have been well documented in the dental literature.1,2 Increasing zones of attached gingiva using palatal donor tissue and the free gingival grafting procedure was introduced by Björn almost a half century ago.3 Using palatal donor tissue in the form of a free soft-tissue autograft for root-coverage procedures was reported by Miller.4 Additional procedures were reported using lateral5 or coronal repositioning6-8 of the adjacent attached gingiva via a pedicle flap or the coronal repositioning of previously grafted tissue.9,10 Miller also reported on gingival grafts placed over root surfaces to correct areas of deep-wide gingival recession.11 Further surgical advancements led to the use of subepithelial connective tissue from the palate to obtain root coverage.12-14 Figure 1 shows the pretreatment view of a mandibular central incisor, and Figure 2 depicts the post-treatment view of the site treated with a subepithelial connective tissue graft harvested from the patient’s palatal tissues.
One of the impediments to patients’ accepting soft-tissue procedures to correct gingival loss is the trauma from harvesting palatal donor tissue. Depending on the volume of tissue required to correct the recession, multiple harvesting procedures may be required. Also, the size of the autogenous graft that can be harvested at a single time is limited, and irregularity in thickness may be difficult to control. Moreover, there may be an inadequate amount of connective tissue present in a shallow palate, and the patient’s medical status may also play a role in his or her candidacy for palatal donor site surgery.
Use of Acellular Dermal Matrix Grafts
As a result of some of these concerns, corrective gingival surgery eventually expanded to include the use of acellular dermal matrix grafts as a substitute for palatal connective tissue grafts.15-17 A major advance in dentistry has been the successful replacement of lost gingiva with acellular dermal matrix grafts. The use of such grafts for the oral rehabilitation of patients has greatly broadened the scope of clinical dentistry, with a major benefit to patients being the avoidance of palatal tissue harvesting. Acellular dermal matrix grafts have an advantage over autogenous subepithelial connective tissue grafts in that there is unlimited availability. Using an allograft allows for inclusion of as many sites as necessary in just one surgical procedure, which not only improves patient case acceptance but allows for the treatment of large-scale cases not previously possible. The surgical procedure is more efficient for the surgeon and less traumatic for the patient,18 and the predictability and long-term success of dermal matrix grafts have been well documented in soft-tissue augmentation around teeth as well as dental implants. The high quality of the donor tissue, its natural esthetic appearance, and patients’ increased acceptance of therapy make this a desirable replacement procedure for palatal soft-tissue grafting.
One of the first decisions clinicians must address regarding soft-tissue grafting is whether or not an acellular dermal matrix would be a better choice than an autogenous graft. Harris reported a comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft and observed no clinical or statistical difference between the two materials.17 Henderson et al reported on predictable multiple-site root coverage using an acellular dermal matrix autograft,19 and there is additional clinical documentation of dermal matrix grafts and their successful use in root-coverage procedures.20-23
Past surgical techniques for root coverage were developed primarily for subepithelial connective-tissue grafts. While reasonable results can be achieved with those methods, they are not as appropriate for acellular dermal matrix grafts, which involve a different healing process than connective tissue grafting due to their different vascular and cellular structures. Although connective tissue and acellular dermal matrix have a slightly different histological appearance, both can successfully be used to cover denuded roots with similar attachments and no adverse healing.18
Thus, the surgical technique developed primarily for the autograft may not be adequate for the allograft. A comparison was done of the clinical results of two surgical techniques24—the “conventional” procedure as developed by Langer and Langer13 originally for subepithelial connective tissue grafts using vertical releasing incisions on the proximal surfaces of the tooth versus a modified procedure for the treatment of localized gingival recession with acellular dermal matrices in which the incision involved papilla preservation with vertical releasing incisions displaced to the mesial and distal line angles of the adjacent teeth, thereby providing a broader flap in order to favor healing to the avascular and acellular tissue for a more adequate blood supply. This modified technique also minimized possible graft exposure, resulting in less root coverage. The researchers concluded that the modified technique is more suitable for root-coverage procedures with the dermal matrix because it had statistically significant better clinical results compared to the traditional technique.24
There are various techniques using acellular dermal matrix for root coverage of gingival recession. Comparison of two of these techniques—one using a broader flap and vertical releasing incisions and one without vertical releasing incisions—showed that both techniques provided significant root coverage, good esthetic results, and similar levels of postoperative discomfort. However, the technique using vertical releasing incisions had statistically significant better results for root coverage of localized gingival recession.25
Allen et al described a tunneling technique in which a surgical pouch is created, the acellular dermal matrix is placed into the pouch, and the pouch is then coronally repositioned to completely cover the graft.26 Comparison of the percentage of root coverage obtained with a coronally positioned flap plus acellular dermal matrix allograft to that of a tunnel technique plus acellular dermal matrix showed that a coronally positioned flap offered greater defect coverage (95%) when compared to acellular dermal matrix coverage (78%).27 Flap procedures provide increased visibility and are clinically easier to manage, while the tunnel technique is more laborious, is clinically challenging to perform, and offers much less access to the surgical site.
Nevertheless, success rates have been reported to vary in different areas of the mouth, in different patients, and with different materials used. Factors such as material biocompatibility, tooth root surface characteristics, surgical technique, and host conditions—such as muscle pulls—have all been shown to influence root coverage success. Consequently, further modifications in surgical techniques will be suggested to increase success. The application of enamel matrix derivatives does not improve the clinical efficacy of acellular dermal matrix in combination with coronally advanced flaps in root coverage procedures.28
Long-term results of subepithelial connective tissue graft versus acellular dermal matrix allograft in the treatment of gingival recession show both techniques are similar in terms of complete root coverage; however, the gingival width does not increase in acellular dermal matrix–treated sites. Increase of gingival width is stable in subepithelial connective tissue graft–treated sites, but reached presurgical values in acellular dermal matrix–treated cases.29 In cases where root coverage and gain in keratinized tissue are expected, the use of subepithelial connective tissue grafts seems to be more appropriate.30 Overall comparisons suggest that subepithelial connective tissue grafts are considered the “gold standard” procedure in the treatment of recession-type defects. The new acellular dermal matrix materials, however, do provide improved patient satisfaction and esthetics, are available in abundance, and lead to reduced postoperative discomfort and surgical time.31
Limited data exist on the changes in esthetic conditions reflected in the opinions and preferences of patients for specific procedures. Predictable root coverage and good color match are considered the major therapeutic end points in the treatment of gingival recession. It was reported that acellular dermal matrix grafts may be successfully used to treat gingival recession in populations with a high degree of melanin pigmentation, as the color match will not be an esthetic issue.32 According to the report, the grafted areas underwent melanization from the sixth month onwards, and complete blending with the adjacent sites was obtained at 1 year.
Product Assessment
Once the clinician has decided to use an acellular dermal matrix graft, the next decision concerns which product to use. Various acellular dermal graft products are available, yet there is little information in the literature comparing their distinctions. This review outlines the differences among five main products (Table 1), including various size and thickness options, as well as unique attributes.
Of the five products represented, AlloDerm® (Biohorizons, Inc., www.biohorizons.com) has been in use for more than 17 years with documented success. To date, Puros® Dermis Allograft Tissue Matrix (Zimmer Dental, www.zimmerdental.com), SureDerm™ (Hiossen, www.hiossen.com), Oracell® (Lifenet Health, www.accesslifenethealth.org), and PerioDerm™ (DENTSPLY Implants, www.dentsplyimplants.us) have case reports documenting their usage.33,34
Barker et al compared the treatment of localized tissue recession when using AlloDerm and Puros Dermis.35 Based on the results of this study, there was no statistical or clinical difference in root coverage, probing depth, or keratinized tissue in coronally advanced flaps for root coverage among the two acellular dermal matrix materials. They determined that both materials were successful in achieving root coverage.
All of the reviewed products are available in two thicknesses for use in various procedures. The thin-size grafts range from 0.25 mm to 1.25 mm and are recommended for root coverage and soft-tissue ridge augmentation. The thick-size grafts range from 0.8 mm to 1.8 mm and are recommended for guided bone regeneration and barrier membrane function. Despite these recommendations, there are instances in which the thicker material may be indicated for root coverage procedures in existing situations of very thin soft tissue. The variation in thickness often dictates which size is indicated upon full hydration. A very thin-size 0.25-mm graft may need to be switched for a thicker piece, just as a graft of 1.8 mm may be too thick for a specific site.
AlloDerm® Regenerative Tissue Matrix
AlloDerm, an acellular dermal matrix derived from donated human skin, undergoes a multistep proprietary process that removes both the epidermis and the cells that can lead to tissue rejection. It has an excellent safety history, having been used in more than 1 million procedures ranging from general, urogenital, orthopedic, and dental surgeries.36 In the procurement and safety process of AlloDerm, tissue is accepted from tissue banks in compliance with the American Association of Tissue Banks (AATB) guidelines. The processing of AlloDerm from donor tissue involves a series of steps that removes the epidermis and cells in the dermis that can lead to graft rejection and/or failure of recipient responses. The extracellular matrix that remains is then put through a proprietary freeze-drying step that preserves the tissue without damage from ice crystal formation. Histologic testing is then done on each final lot to verify cell removal.
Puros® Dermis Allograft Tissue Matrix
Puros Dermis is recovered following the rigorous standards of both the Food and Drug Administration and the AATB with either a scalpel or dermatome from the back of the thighs of the deceased donor. The tissue is recovered within 24 hours of death, by a recovery team using an aseptic process that meets the standards set by the AATB. The tissue enters the Tutoplast® tissue sterilization process (Tutogen Medical/RTI Biologics, Inc., www.rtix.com) only after it passes serological tests, such as those for human immunodeficiency virus, hepatitis, human T-lymphotropicvirus, and syphilis. The multistep Tutoplast process removes all antigenicity, inactivates all kinds of pathogens, preserves tissue structure and collagen, preserves biomechanics, guarantees sterility, and results in graft healing comparable to autografts. The process itself consists of donor selection, osmotic treatment, oxidative treatment, alkaline treatment (different from bone), and solvent dehydration. Tutoplast processing of Puros Dermis involves a limited-dose gamma irradiation, which provides a sterility level of 10-6 and preserves graft integrity. The Puros Dermis tissue allograft has a strength-to-failure measurement of 5 pounds ± 0.8 pounds, can be stored at room temperature, has a 5-year shelf-life, and, due to the Tutoplast process, has no residual chemicals when it is packed and delivered to the clinician.37
PerioDerm™ Acellular Dermis
PerioDerm Acellular Dermis undergoes a three-phase process that gently cleans, decellularizes, and disinfects without cross-linking or compromising the integrity of the dermal matrix. Each piece is quality-controlled for 90%+ uniformity in thickness. Biologic integrity is maximized via a proprietary process that avoids high-dose gamma irradiation. PerioDerm is aseptically processed by the Musculoskeletal Transplant Foundation and is rendered sterile per United States Pharmacopeia Standard 71 (USP 71). PerioDerm has a shorter hydration period, no need for refrigeration, no added antibiotics, and a 3-year shelf-life.
Oracell®
Oracell is decellularized with LifeNet Health’s patented technology called Matracell™, which renders the product acellular without compromising biomechanical or biologic properties. More than 97% of the DNA is removed from the dermis, so immunogenic potential is low. Oracell is infused with glycerin-based solution to replace rehydration at chairside.
SureDerm™
SureDerm is transported in ambient temperature, but requires refrigeration to preserve better shelf-life. It is packaged in antibiotics and requires a separate rehydration rinse similar to AlloDerm.
Laser-Assisted Vestibuloplasty Approach (LAVA)
The authors have introduced a laser-assisted vestibuloplasty approach (LAVA) procedure that is designed to prevent recurrent recession after surgical correction, which has been reported in the literature.29,38 This first involves a flap procedure for insertion of the dermal matrix graft material to be used to achieve root coverage and increase zones of existing attached keratinized tissues. To complete the vestibuloplasty procedure, the surgeon separates the mucosal tissues apical to the preexisting mucogingival junction in the area of the teeth exhibiting gingival recession. The authors advocate using a Nd:Yag laser with a power setting at 5 watts to accomplish the vestibuloplasty and release any excessive muscle attachments or frenal pulls in the affected areas (Figure 3).
The flap approach follows an incision design where the crest of the papilla is preserved and a V-type incision is made in the papillary tissues (Figure 4). This allows the preexisting papillary tissues in the area just inferior to the contact point of the natural teeth affected to be used as a tissue bed to provide vascularization to the flap/dermis complex. Prior to securing the dermal matrix tissue, the papillary tissues that remain after the incision technique outlined must be de-keratinized by using a #4 round diamond bur. This allows for the introduction of initial blood flow and vascularization of the dermal matrix tissues and nourishment of the coronally repositioned flap in the critical area of the interproximal papillary tissues. After securing the dermal matrix tissue from the palatal/lingual aspect of the teeth being treated and coronally repositioning the buccal flap, closure is achieved using an interrupted sling suturing technique.
Once the entire surgical site has been closed, periosteal securing sutures, which secure the tissues repositioned superior to the reestablished mucogingival junction, complete the LAVA approach (Figure 5). These securing sutures will prevent any pull on the tissues coronally repositioned in the initial healing phase once the patient resumes normal function in the postoperative period.
Case Report
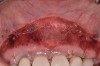
A 24-year-old, nonsmoking man presented for correction of gingival clefting and recession in the maxillary left anterior sextant (Figure 6). The patient’s medical history was noncontributory. After consultation and presenting various options to the patient to correct the localized recession, the patient opted for the use of acellular dermal matrix tissue rather than harvesting tissue from his palatal area.
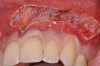
After administration of an appropriate local anesthetic, a laser-assisted split-thickness vestibuloplasty was performed superior to the mucogingival junction from the right central incisor to the left first premolar region (Figure 7). Prior to any incisions being performed, a 20-mm x 40-mm dermal matrix graft (Puros Dermis), a 0.8-mm to 1.7-mm thick piece of tissue, was trimmed to appropriate length and width to correct the gingival defect present (Figure 8). The tissue graft was sized to 6 mm in height and spanned to the mesial line angle of the left central incisor to the distal line angle of the left canine area. The dermal matrix graft was rehydrated with the patient’s own platelet-rich plasma solution, which was harvested from 20 cc of whole blood collected immediately prior to the surgical procedure (Figure 9).
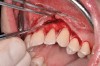
After initial incision techniques were done, a papillary-sparing incision was performed, leaving the crest of the papilla intact (Figure 10), thus allowing flap closure to be accomplished with a bed of autogenous tissue to support revascularization of the dermis/buccal flap complex. A full-thickness flap was then elevated to the mucogingival junction, after which a split-thickness dissection with a blunt elevator superior to the mucogingival junction area was performed to allow for release of tension and to let the buccal flap be mobilized coronally at closure (Figure 10). Care must be taken to avoid perforating the tissue in the region of the vestibuloplasty incision. After tension-free release of the flap, root planing of the exposed root surfaces was accomplished, followed by removal of the smear layer of cementum and chemical detoxification with a citric acid solution pH1 applied over the previously root-planed surfaces. After root preparation, the keratinized papillary tissues that remained following initial incisions were de-keratinized by using a #4 round diamond on a high-speed handpiece. De-keratinization provided a wound bed that enabled the dermal matrix graft to be secured and supported revascularization of the coronally repositioned buccal flap at closure.
The platelet-rich plasma-enriched acellular dermal matrix graft was then secured by a continuous sling suturing technique with a 6.0 polypropylene suture (Surgical Specialties Corp., www.surgicalspecialties.com), initiated on the palatal aspect of the maxillary left canine area, and continued anteriorly to the mesial of the maxillary left central incisor. The suture was then tied off on the palatal aspect of the maxillary central incisor (Figure 11). This palatal suturing technique allows for easy removal of the dermal matrix–securing suture from the palatal aspect 1-month postoperatively, without disruption of the buccal maturing tissues.
Closure was then accomplished by coronally repositioning the buccal flap and securing the flap using an interrupted sling suturing technique with 5.0 Monocryl™ (Ethicon, www.ethicon.com). The interrupted sling suture technique was started at the mesial aspect of each tooth in the area of the mucogingival junction, and passed interproximally to the distal aspect of the same tooth; the flap was then engaged in the mucogingival junction area, and the suture was passed back interproximally at the distal aspect palatally, then to the buccal from the mesial aspect of the tooth. The suture was then tied off at the mesial aspect. This closure technique was performed for each of the affected tooth sites (Figure 12 and Figure 13).
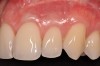
Once the flap was coronally repositioned and secured, a securing periosteal tac procedure was performed to prevent any micro movement superior to the mucogingival junction in the initial healing phase. A 4.0 chromic gut suture (Surgical Specialties Corp.) was used to engage the periosteum superior to the mucogingival junction and stabilize the tissues superior to the securing suture (Figure 12 and Figure 13). Multiple periosteal tac sutures may be necessary, depending on the size of the surgical field. Postoperatively, the periosteal tac sutures are routinely removed if not totally absorbed at 7 days; the flap sutures are removed at 2 to 3 weeks, and dermal-securing sutures are removed 1 month after surgery. The 1-year post-treatment clinical view shown in Figure 14 depicts the excellent soft-tissue result, band of attached–keratinized tissue present, and excellent color match to the host gingival tissues.
Conclusion
Adequate zones of keratinized, attached tissue are important for long-term periodontal health and maintenance. Restorative and/or cosmetic dental procedures benefit from this type of periodontal environment. Soft-tissue grafting and augmentation procedures have been developed and perfected over the past 30 years. Incorporation of acellular dermal matrix grafts have simplified the procedure, making it more patient friendly and enabling patients who have avoided palatal donor harvesting to proceed with the procedure using a safe and effective biomaterial.18 Acellular dermal matrix tissue has proven to be equal to palatal connective tissue for root coverage procedures in randomized, controlled clinical studies.10,14,16
Dermal matrix grafting possesses distinct advantages compared to palatal connective tissue, including: avoidance of the palatal donor surgical site; multiple teeth treatment at one visit; unlimited amounts of donor tissue availability; high quality of donor tissue; and the ability to match or improve upon the results observed with autogenous palatal tissue grafts. Additionally, it offers a higher case acceptance rate and less postoperative discomfort.
Reestablishing the proper soft-tissue quality with acellular dermal matrix grafts provides a safe, reliable option to palatal donor connective tissue for root coverage. A new surgical procedure has been outlined to aid in the prevention of recurrent gingival recession by increasing the vestibular depth in the treated area and reestablishing muscle attachments apical to the treated surgical site. By incorporating this split-thickness vestibuloplasty approach, the supply of blood to the main portion of the flap is maintained, thereby providing an adequate blood supply to reestablish vascularization of the dermal matrix tissue and the coronally advanced buccal flap. Additionally, in the LAVA procedure, the manual stabilization of the repositioned coronal flap with the periosteal tacking sutures prevents movement of the coronal portion of the flap and dermis complex, which prevents micro movement of the graft complex in the early postoperative healing phase. This, in turn, allows for a simplified surgical procedure, as compared to the more technically challenging tunnel graft technique, and offers greater flap security and stability compared to that of the flap root coverage procedure without vestibuloplasty and periosteal stabilizing sutures. Additional clinical trials are recommended to validate the claims made by the authors in regards to the efficacy of the LAVA procedure.
DISCLOSURE
The authors declare no financial interest in any of the products mentioned in this article.
ABOUT THE AUTHORS
Jennifer T. Silc, DDS, MS
Private Practice, Schaumburg, Illinois
Paul S. Petrungaro, DDS, MS
Private Practice, Chicago, Illinois
REFERENCES
1. Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part II. Prosthetic/periodontal interrelationships. Compend Contin Educ Dent. 1983;4(6):549-562.
2. Garber DA, Rosenberg ES. The edentulous ridge in fixed prosthodontics. Compend Contin Educ Dent. 1981;2(4):212-223.
3. Björn H. Free transplantation of gingival propria. Sven Tandlak Tidskr. 1963;22:684-689.
4. Miller PD Jr. Root coverage using a free soft tissue autograft following citric acid application. Part 1: Technique. Int J Periodontics Restorative Dent. 1982;2(1):65-70.
5. Björn H. Coverage of denuded root surfaces with a lateral sliding flap. Use of free gingival grafts. Odontol Revy. 1971;22(1):37-44.
6. Guinard EA, Caffesse RG. Treatment of localized gingival recessions. Part III. Comparison of results obtained with lateral sliding and coronally repositioned flaps. J Periodontol. 1978;49(9):457-461.
7. Tarnow DP. Semilunar coronally repositioned flap. J Clin Periodontol. 1986;13(3):182-185.
8. Allen EP, Miller PD Jr. Coronal positioning of existing gingiva: short term results in the treatment of shallow marginal tissue recession. J Periodontol. 1989;60(6):316-319.
9. Bernimoulin JP, Lüscher B, Mühlemann HR. Coronally repositioned periodontal flap. Clinical evaluation after one year. J Clin Periodontol. 1975;(2)1:1-13.
10. Matter J. Free gingival graft and coronally repositioned flap. A 2-year follow-up report. J Clin Periodontol. 1979;6(6):437-442.
11. Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5(2):14-37.
12. Raetzke PB. Covering localized areas of root exposure employing the “envelope” technique. J Periodontol. 1985;56(7):397-402.
13. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56(12):715-720.
14. Langer B, Calagna LJ. The subepithelial connective tissue graft. A new approach to the enhancement of anterior cosmetics. Int J Periodontics Restorative Dent. 1982;2(2):22-33.
15. Silverstein LH, Callan DP. An acellular dermal matrix allograft substitute for palatal donor tissue. Post Grad Dentistry. 1997;3(4):14-21.
16. Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Periodontol. 1998;69(11):1305-1311.
17. Harris RJ. A comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft: results of 107 recession defects in 50 consecutively treated patients. Int J Periodontics Restorative Dent. 2000;20(1):51-59.
18. Allen EP. AlloDerm: an effective alternative to palatal donor tissue for treatment of gingival recession. Dent Today. 2006;25(1):48-52.
19. Henderson RD, Greenwell H, Drisko C, et al. Predictable multiple site root coverage using an acellular dermal matrix allograft. J Periodontol. 2001;72(5):571-582.
20. Paolantonio M, Dolci M, Esposito P, et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: a comparative 1-year clinical study. J Periodontol. 2002;73(11):1299-1307.
21. Novaes AB Jr, Grisi DC, Molina GO, et al. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001;72(11):1477-1484.
22. Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: a comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73(12):1405-1411.
23. Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72(8):998-1005.
24. Barros RR, Novaes AB, Grisi MF, et al. A 6-month comparative clinical study of a conventional and a new surgical approach for root coverage with acellular dermal matrix. J Periodontol. 2004;75(10):1350-1356.
25. Felipe ME, Andrade PF, Grisi MF, et al. Comparison of two surgical procedures for use of the acellular dermal matrix graft in the treatment of gingival recessions: a randomized controlled clinical study. J Periodontol. 2007;78(7):1209-1217.
26. Cummings LC, Kaldahl WB, Allen EP. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J Periodontol. 2005;76(2):178-186.
27. Papageorgakopoulos G, Greenwell H, Hill M, et al. Root coverage using acellular dermal matrix and comparing a coronally positioned tunnel to a coronally positioned flap approach. J Periodontol. 2008;79(6):1022-1030.
28. Pourabbas R, Chitsazi MT, Kosarieh E, Olyaee P. Coronally advanced flap in combination with acellular dermal matrix with or without enamel matrix derivatives for root coverage. Indian J Dent Res. 2009;20(3):320-325.
29. Moslemi N, Mousavi Jazi M, Haghighati F, et al. Acellular dermal matrix allograft versus subepithelial connective tissue graft in treatment of gingival recessions: a 5-year randomized clinical study. J Clin Periodontol. 2011;38(12):1122-1129.
30. Chambrone L, Chambrone D, Pustiglioni FE, et al. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J Dent. 2008;36(9):659-671.
31. Fu JH, Su CY, Wang HL. Esthetic soft tissue management for teeth and implants. J Evid Based Dent Pract. 2012;12(3 suppl):129-142.
32. Shanmugam M, Sivakumar V, Anitha V, Sivakumar B. Clinical evaluation of alloderm for root coverage and colour match. J Indian Soc Periodontol. 2012;16(2):218-223.
33. Petrungaro PS. Acellular dermal matrix tissue grafts—correcting insufficient zones of keratinized tissue before esthetic, reconstructive, and implant procedures with acellular dermal grafting materials. Inside Dentistry. 2010;6(1):34-44.
34. Petrungaro PS. Correcting soft tissue deficiencies prior to esthetic dental procedures. Journal of Cosmetic Dentistry. Fall Special Issue 2009; 25(3):129-135.
35. Barker TS, Cueva MA, Rivera-Hidalgo F, et al. A comparative study of root coverage using two different acellular dermal matrix products. J Periodontol. 2010;81(11):1596-1603.
36. Data on file. LifeCell Corporation. Branchburg, NJ. www.lifecell.com/health-care-professionals/lifecell-products/allodermr-regenerative-tissue-matrix/. Accessed April 17, 2013.
37. Data on file. Tutogen Medical/RTI Biologics, Inc. Alcoa, FL. www.rtix.com. Accessed April 17, 2013.
38. Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75(5):734-743.