You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
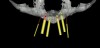
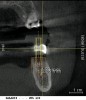
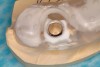
From a surgical perspective, the highest priority is the protection of vital anatomic structures from the trauma of surgery. When performing implant placement in the posterior mandible, it is critical to avoid trauma to the inferior alveolar neurovascular bundle (inferior alveolar canal) and prevent perforation of the lingual cortex. In a radiographic study, evaluating human CT scans, Quirynen et al reported on the variations of mandibular anatomy 4 mm to 6 mm anterior to the mental foramen.1 These authors introduced three classifications, based on the geometry of the mandibular bone and presence of lingual concavities and inclinations. Knowledge of the local anatomic situations in proposed implant sites can prevent potentially catastrophic injury during implant insertion. A CT scan affords the surgeon the opportunity to measure and appreciate the locations of these structures, but not the vertical and horizontal drill control necessary to be absolutely certain that inadvertent over-preparation or deviations do not occur. Many manufacturers sell so-called "drill stops," but these offer little horizontal control in softer bone types. The fabrication of a surgical guide, stabilized by either teeth or fixed via fixation screws onto the surrounding alveolar ridge or mucosa, affords the surgeon an opportunity to perform surgery based on planning software and a "sleeve-in-sleeve" system to control horizontal and vertical limits of the twist drills used for osteotomy preparation. Using this concept, Horowitz et al2 demonstrated small angular and vertical deviations from planned implant positions in vitro. This is demonstrated by the first clinical example. A cone-beam CT scan allows the surgeon to locate the 3-dimensional location of the IAC and lingual concavity of the mandibular alveolus (Figure 1 and Figure 2). Next, implant positions based on the prosthetic treatment plan and translated to the scan via a barium-containing scanning appliance (Figure 3), are planned via various planning software. Sarment et al3 compared the accuracy of implant placement between a "standard" surgical guide and a stereolithic guide by comparing the spatial deviations between virtual and actual placement in mandibular models. The control guide resulted in coronal deviations of 1.5 mm +/- 0.7 mm and 2.1 mm +/- 0.97 mm at the apical end. The use of the stereolithic guide produced coronal deviations of 0.9 mm +/- 0.5 mm and apical deviations of 1 mm +/- 0.6 mm. The authors concluded that this type of surgical guide can improve the accuracy of implant placement. In a study evaluating the accuracy of implant placement using an integrated system of guidance, Dreiseidler4 found crestal deviations of 217 µm +/- 99 µm and apical deviations of 343 µm +/- 146 µm when comparing planned and actual placements on a partially edentulous model. They also reported on an axial deviation of 1.09° +/-0.51°.
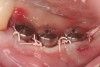
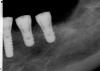
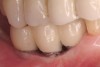
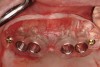
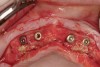
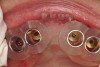
A surgical guide, based on the 3-dimensional implant locations is fabricated for use in conjunction with the selected implant manufacturer's guided surgery instrumentation (Figure 4). Using this template, safe implant positioning in all three dimensions is performed, avoiding trauma to vital anatomic structures respecting the restorative outcome prescribed prior to surgery (Figure 5 and Figure 6). Following 8 weeks of transmucosal healing time, standard restorative therapy is initiated (Figure 7).
Optimization of Available Bone for Osseointegration
For many edentulous patients, advanced resorption of the alveolar ridge has occurred, leading to poor function of their existing complete dentures. Regardless of the manner of extraction, Araujo et al5 demonstrated significant alveolar bone loss in horizontal dimensions at 6 months after tooth removal in the canine model. The treatment of these patients with implant therapy to provide anchorage for new, removable prostheses is being recommended with almost routine frequency in clinical practice. Some implant manufacturers are advocating forms of guided implant placement, coupled with delivery of prefabricated prostheses. These procedures can be performed in either an open or closed (flapless) manner. Katsoulis et al6 investigated the efficacy of this modality for theoretical fixed and removable prosthetic solutions for 40 patients presenting with edentulous maxillas.
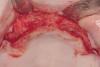
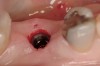
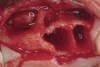
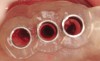
These patients were tested for two different treatment options; either a bar-supported overdenture requiring four implants in the first premolar area or anterior to the bicuspids, or a "simple fixed prosthesis" anchored by six implants placed bilaterally from first-molar-to-first-molar regions. When a flapless, guided surgery was contemplated, the investigators determined that based on the available maxillary bone, 70% of patients (28 implants) could undergo this treatment modality. Of the 40 patients, 15% could not have any implants placed due to inadequate bone height and width. For "simple fixed prostheses," only 30% (72 implants) of patients presented with adequate bone dimensions permitting a flapless guided placement of six maxillary implants placed symmetrically around their arch in a flapless manner. For the fixed treatment, 18% of patients could not have any implants placed due in insufficient bone dimensions. The authors concluded that advertisement of computer-guided, flapless solutions are "euphoric," and tend to overlook patients' individual oral and anatomic conditions. They felt that guided surgery does provide better control of implant placement leading to higher predictability of treatment outcomes. In a study where the accuracy of virtually placed implants and actual positions in a cadaver model were compared, Petterson et al7 found significant differences related to deviations in hex, apex, and depth of the two placements. Without the preparedness to temper treatment expectations or plan on delivery of the pre-fabricated "final" prosthesis immediately after surgery, these types of studies should lead clinicians to proceed with caution in these procedures. The efficacy of an open approach, one requiring a mucoperiosteal flap, may be improved upon with a guided approach in cases where severe ridge-resorption is treated, and may be more predictably handled using this technology. A bone-supported template, affixed to the alveolar ridge with fixation screws, can be fabricated with numerous software package and stereolithography (Figure 8 and Figure 9). A full-thickness flap is elevated, exposing the resorbed alveolar ridge (Figure 10). Using osseous fixation screws, the template is stabilized to the ridge, preventing any movement during osteotomy preparation (Figure 11). Using a sleeve-in-sleeve, manufacturer-specific surgical armamentarium, implants can be placed in the optimal positions, where the maximum amount of initial bone to implant contact is achieved. The anticipated bone augmentation procedures can then be performed, which is not possible with a closed procedure (Figure 12). The flaps are subsequently closed, allowing for the patient to wear any transitional, soft-relined denture. The surgical guide can also serve an important function at the time of implant uncovering. The location of the implants beneath the healed mucosa can be determined by seating the guide and sounding with a 30-gauge anesthetic needle. Using a tissue punch, the implants can be uncovered without flap elevation; the cover screws can be removed and the appropriately sized healing abutments can be placed (Figure 13). From this point forward, traditional prosthetic steps are followed to fabricate an implant-supported/retained overdenture.
Minimally Invasive Flapless Surgery
For many patients, one of the biggest objections to implant therapy is the prospect of having a "surgical" procedure. With the safety of implant placement already discussed in this article, it is possible, under conditions where adequate hard tissue as well as keratinized mucosa are present, to avoid the elevation of a mucoperiosteal flap to position endosseous implants correctly. This minimally invasive approach is very appealing to patients and surgeons alike. Many doctors are routinely performing flapless surgery in many if not all implant placement procedures. This should be a cause for concern. In an in vitro study, Van de Velde et al8 exposed serious possible complications with routine use of flapless implant placement. Clinicians, including specialists, general practitioners, and dental students, were required to place implants on models in molar, bicuspid, and incisor locations in a flapless manner. Artificial mucosa covered the osteotomy sites, and clinicians were afforded a cross-sectional, CT scan view of the receptor site to aid in implant positioning. When the artificial mucosa was removed, implant malpositioning occurred in 59.7% of osteotomy preparations. It is clear from this study that without a surgical guide, based on tomographic data and planning software, that blind or flapless surgery should be undertaken with extreme caution.
Interestingly, the same group of investigators (Van de Velde8) compared guided-placement coupled with immediate loading to conventional, open-placement, and delayed loading. In posterior maxillary sites in partially edentulous patients, similar levels of bone remodeling at 3 months loading existed in both groups of patients. The failure rate in the test group was 2.7%, related to one implant, which supported a fractured provisional bridge. The authors speculated that the cause for failure was mechanical overload in the posterior region of an un-splinted implant. None of the conventionally placed and conventionally loaded implants failed at 3 months post-loading. The conclusions of the authors were that when clinicians fail to obtain CT scans with patients wearing radiopaque scanning appliances and digital planning of implant placement with respect to restorative considerations and local anatomy, flapless placement is a "guessing game" increasing the risks for "suboptimal" outcomes. They felt that with careful planning, using simulation software and the resulting surgical guides, implants could be predictably installed with a flapless approach. An example of when this approach can be implemented is the site of previous extraction(s) where augmentation procedures are implemented to prevent ridge resorption. After sufficient time for healing and bone regeneration has occurred, a CT scan can be obtained with the patient wearing a radiopaque scanning appliance (Figure 14). Either a second template, fabricated with planning software, can be made, or the scanning appliance can be converted into a surgical guide. Various companies offer different options for surgical stent fabrication.
A tissue punch can be used to expose the selected site for osteotomy preparation, followed by securing a tooth-borne surgical guide (Figure 15). Then the implant system-specific guided surgery protocol is followed to accurately place the implant through the mucosa (Figure 16). A healing cap is placed, and there is usually not a need for sutures to achieve hemostasis.
Another opportunity to implement flapless, guided implant surgery is when a site of previous sinus grafting is ready for implant installation. These are situations where the patients have already undergone significant surgery to reconstruct bone lost secondary to maxillary posterior tooth loss. This procedure requires wide flap-reflection and invasive treatment to elevate the antral floor (Figure 17). Often these sites heal with a wide band of keratinized mucosa and adequate bone available for implant placement. If there is not need to buccally reposition keratinized mucosa for peri-implant maintenance, it may not be justified to raise a second mucoperiosteal flap in these areas to insert dental implants. As stated by Van de Velde,9 the flapless placement of dental implants without taking appropriate diagnostic steps and fabrication of a guided-placement surgical guide can become a "guessing game." With the use of a guided surgery template, the overlying mucosa can be opened via a tissue punch and implants placed in a safe and accurate manner. This will lead to significantly less postoperative discomfort, hemorrhage, and edema. The flapless approach will also result in little to no soft tissue recession around the implants.
Not only does guided surgery result in safe and accurate implant placement, but by predetermining the path of implant insertion, the bone density of the osseous receptor site can be predetermined, which may affect the surgical procedure. In softer bone, clinicians will often under-prepare the osteotomy dimensions to increase the likelihood of achieving primary implant stabilization. Using Hounsfield units to estimate bone density, the surgeon can begin a surgical procedure with increased knowledge of the qualitative bone density prior to starting osteotomy preparation. Misch10 classified bone density by radiodensity numerical values to assist in approaching surgical implant placements. The bone density of each millimeter of osteotomy depth can be determined with planning software, giving the surgeon greater insight to the proposed implant bed (Figure 19). Turkyilmaz and McGlumphy11 found a clinical correlation between Hounsfield units measured on CT scans of proposed implant locations, insertion torque, and implant stability (ISQ). The radiodensity of sites of failed implants was consistently lower than those sites of successful implants. The authors concluded that a non-invasive method, such as CT scanning, may be used prior to implant surgery, and may allow clinicians to plan on modifying their approach before starting active therapy. Most implant planning software is capable of measuring bone density via Hounsfield units, which makes the realization of drill resistance prior to surgery a possibility for clinicians today.
The Esthetic Zone
In the area of implant esthetics, much has been made of the inter-implant distance and positioning of implants at a critical horizontal distance from neighboring teeth. The belief is that the presence or absence of proximal papillae is dependent on the height of bone in these areas. Tarnow12 and colleagues demonstrated radiographically that in bone-level implants with a "butt-joint" prosthetic connection that the critical distance between adjacent implants, capable of supporting an inter-implant papilla, is about 3 mm. This is to compensate for approximately 1.5 mm of horizontal bone remodeling subsequent to formation of the biologic width around these types of implants. Jung13 radiographically and Cochran14 histologically showed that by modifying the prosthetic connection at the implant platform and by shifting the abutment-implant interface horizontally toward the center of the fixture, further from the osseous crest, the vertical and horizontal bone remodeling is minimized. Canullo15 showed in a human study that this phenomenon could minimize bone remodeling. The conclusions drawn from these studies is that the critical inter-implant distance, though yet to be determined, may be less than the 3 mm once thought to be mandatory in achieving esthetic outcomes.
Another area of crucial importance regarding implant positioning in the esthetic zone is the facial-palatal placement and the presence or absence of facial bone. Though it was once considered a modality of preserving the original, pre-extraction alveolar dimensions, Araujo et al16 demonstrated in the dog model that implant placement failed to prevent remodeling of extraction sockets at 3 months. Others, such as Buser et al,17 advocate guided bone regeneration (GBR) to achieve 2-mm to 3-mm thick facial bone to provide long-lasting support for facial soft tissue. In sites where extraction and augmentation of the sockets has occurred, it is of great importance not to place the implants in a facial position, which could lead to bone loss, which could then result in soft tissue recession and esthetic failure. This opportunity to place implants into augmented, anterior sites also gives the surgeon the chance to perform minimally invasive, and sometimes flapless, implant placement. Rimer18 was quite critical, and rightfully so, of overuse of some manufacturer-recommended implant treatments. He was, however, in favor of the use of this technology to increase patient comfort with a less invasive procedure.
In esthetically sensitive areas of the dentition, precise implant positioning can be the most critical factor in achieving acceptable results for patients. No area can be more amenable to the accuracy of guided placement. Planning software is capable of measuring the distances of implants from adjacent teeth, as well as inter-implant distances (Figure 20). Whether the prosthetic connection of the proposed implants are of a "platform-shifted" or "butt-joint" nature, these distances, which can determine whether esthetic treatment outcomes are acceptable or not, can be pre-determined by combined evaluation of diagnostic wax-up, virtual implant and prosthetic design, and placement within various software packages prior to executing any type of surgical procedures. When 3-dimensional positioning of the implants is pre-planned and both the oral surgeon and restorative dentist are satisfied that anatomic and esthetic principles have been optimized "virtually," a surgical guide can be fabricated to predictably place the planned implants into these pre-determined positions. This will take much of the guesswork out of traditional implant therapy, and give patients and clinicians greater confidence in the outcome of treatment.
Conclusion
Practically speaking, one of the goals of all surgeons should be to reduce patient morbidity associated with all surgical procedures. The nature of individual surgeries may or may not afford clinicians flexibility related to choice of technique. If favorable anatomic conditions exist, flapless implant placement may reduce postoperative pain and swelling. In a study comparing bone- and mucosa-supported guides, Arisan et al19 found surgery using mucosa-supported guides reduced the duration of surgery, pain intensity, and the need for postoperative analgesics. These authors point out drawbacks to both modalities and emphasize careful case selection. Not only is it crucial to provide short-term benefits of simplified procedures, but also long-term successful outcomes must be assured before the incorporation of these "newer" modalities into clinical practice. In a limited cohort study, Barter et al20 demonstrated 97.7% implant survival 60 months post-placement of implants with a computer-guided, flapless approach. It is important to point out that these patients underwent extensive ridge and sinus grafting procedures 4 to 5 months prior to implant insertion. By providing favorable anatomic situations surgically, these patients benefitted from the elimination of a second extensive and invasive surgery to place the implants.
Several consensus papers, critical of the literature, have reviewed computer-guided, implant surgery. Schneider et al21 performed a systematic review on the accuracy and clinical outcomes of this modality, looking exclusively at static template-based implant dentistry. These authors found the occurrence of surgical complications to be 9.1% of the 428 patients treated in their reviewed studies. Limited inter-occlusal distance in posterior segments was the most often reported complication in 10 patients (2.3%). They found the survival rate of implants placed with computer-guided technology comparable to conventionally placed implants, ranging from 91% to 100% after 1 to 5 years of observation time. Jung et al22 also reviewed the literature to draw key conclusions related to computer technology applications in surgical implant dentistry. When they evaluated studies testing surgical guides, the mean error at implant entry point was 1.12 mm and 1.2 mm at the apex. Interestingly, the error for implants placed with dynamic navigation systems were about half of those found with a static template. They found in the five clinical studies evaluated, a mean failure rate of 3.36% for implants loaded for 12 months.
It can be drawn for the literature and clinical practice that there is a place for computer-guided implant surgery. In areas where vital anatomic structures are at risk of trauma, this treatment modality affords clinicians greater confidence and patients a higher level of safety. In sites of limited bone volume, optimization of available osseous tissue for implant stabilization and potential osseointegration can be maximized with the precise placement of implants using a computer-generated guide. When ideal hard and soft tissues are present, the possibility of performing a minimally invasive or flapless surgery is enhanced with the accuracy a computer-guided placement affords. And finally, the precision that planning software provides in positioning implants virtually in esthetically critical sites can enhance cosmetic outcomes. Many implant manufacturers market this technology as a means to bypass the surgeon requiring the knowledge and skill necessary to perform more traditional or complex surgery. It is the author's opinion, and that shared by many investigators, that this technology should serve as a means to "compliment" the existing and time-tested surgical modalities already commonly used by specialists. The present article aims to point out how computer-guided surgery can be used in specific situations to enhance patient outcomes. It is not indicated at this time, to be used in every type of implant placement. Considerations must be given to the economic, practical, and legitimate needs of each patient and situation. With future research geared toward improving accuracy and ease of operator use, computer-guided surgery should continue to positively effect clinician and patient treatment outcomes.
References
1. Quirynen M, Mraiwa N, van Steenberghe D, Jacobs R. Morphology and dimensions of the mandibular jaw bone in the interforaminal region in patients requiring implants in the distal areas. Clin Oral Impl Res. 2003;14:280-285.
2. Horowitz J, Zuabi O, Machtei EE. Accuracy of a computer tomography-guided template assisted implant placement system: an in vitro study. Clin Oral Impl Res. 2009;20(10):1156-1162.
3. Sarment DP, Sukovic P, Clinthorne N. Accuracy of implant placement with a stereolithic surgical guide. J Oral Maxillofac Implants. 2003;18:571-577.
4. Dreiseidler T, Neugebauer J, Ritter L, et al. Accuracy of a newly developed integrated system for dental implant planning. Clin Oral Impl Res. 2009;20:1191-1199.
5. Araujo MG, Lindhe J. Ridge alterations following tooth extraction with and without flap elevation: an experimental study in the dog. Clin Oral Impl Res. 2009;20(6):545-549.
6. Katsoulis J, Pazera P, Mericske-Stern R. Prosthetically driven, computer-guided implant planning for the edentulous maxilla: a model study. Clin Implant Dent Relat Res. 2009;11(3):238-245.
7. Pettersson A, Kero T, Gilot L, et al. Accuracy of CAD/CAM-guided surgical template implant surgery on human cadavers: Part I. J Prosthet Dent. 2010;103(6):334-342.
8. Van de Velde T, Glor F, De Bruyn H. A model study on flapless implant placement by clinicians with a different level in implant surgery. Clin Oral Implants Res. 2008;19(1):66-72.
9. Van de Velde T, Sennerby L, De Bruyn H. The clinical and radiographic outcome of implants placed in the posterior maxilla with a guided flapless approach and immediately restored with a provisional rehabilitation: a randomized clinical trial. Clin Oral Implants Res. 2010;21(11):1223-1233.
10. Misch CE, Density of bone: effect on surgical approach, healing, and progressive bone loading. Int J Oral Implantol. 1990;6(2):23-31.
11. Turkyilmaz I, McGlumphy EA. Influence of bone density on implant stability parameters and implant success: a retrospective clinical study. BMC Oral Health. 2008;24(8):32.
12. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol. 2000;71(4):546-549.
13. Jung RE, Jones AA, Higginbottom FL, et al. The influence of non-matching implant and abutment diameters on radiographic crestal bone levels in dogs. J Periodontol. 2008;79(2):260-270.
14. Cochran DL, Bosshardt DD, Grize L, et al. Bone response to loaded implants with non-matching implant-abutment diameters in the canine mandible. J Periodontol. 2009;80(4):609-617.
15. Canullo L, Fedele GR, Iannello G, Jepsen S. Platform switching and marginal bone-level alterations: the results of a randomized-controlled trial. Clin Oral Implants Res. 2010;21(1):115-121.
16. Araújo MG, Sukekava F, Wennström JL, Lindhe JJ. Ridge alterations following implant replacement in fresh extraction sockets: an experimental study in the dog. Clin Periodontol. 2005;32(6);645-652.
17. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Impl. 2004;19(Suppl):43-61.
18. Rimer S. COMMENTARY. Immediate loading of the maxilla with prefabricated interim prosthesis using interactive planning software, and CAD/CAM rehabilitation with definitive zirconia prosthesis: 2-year clinical follow up. J Esthet Restor Dent. 2010;22(4):233-234.
19. Arisan V, Karabuda CZ, Ozdemir T. Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin Oral Impl Res. 2010;21:980-988.
20. Barter S. Computer-aided implant placement in the reconstruction of a severely resorbed maxilla: A 5-year clinical study. Int J Periodontics Restorative Dent. 2010;30:627-637.
21. Schneider D, Marquardt P, Zwahlen M, Jung RE. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin Oral Implants Res. 2009;20(Suppl 4):73-86.
22. Jung RE, Schneider D, Ganeles J, Wismeijer D, et al. Computer technology applications in surgical implant dentistry: a systematic review. Int J Oral Maxillofac Implants. 2009;24(Suppl):92-109.
About the Author
Barry P. Levin, DMD
Private Practice
Elkins Park, Pennsylvania
Clinical Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania
Diplomate, American Board of Periodontology