You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Root coverage is dependent on the height of the adjacent papilla. The coronal apex of the papilla will dictate where the gingival margin can be placed. Patients who have lost interdental papilla may be poor candidates for predictable root coverage. Additionally, the recession may be confined to the attached gingiva or may extend beyond the mucogingival junction.1 In general, Miller class I and II defects in which the interdental tissue has been maintained may allow complete root coverage. When interdental tissue has been lost (Miller class III and IV defects), achieving complete root coverage may not be possible.
Methods and materials
Preoperative
It is recommended that preoperative administration of a broad-spectrum antibiotic should be initiated beginning the day before surgery and continued for 5 to 7 days postsurgery. Additionally, if not contraindicated by the patient’s medical history, administration of a nonsteroidal anti-inflammatory drug (NSAID) should be administered 1 hour before surgery. The NSAID will help depress prostaglandin synthesis elicited during the surgery and help mediate postoperative discomfort and associated swelling. Table 1 lists the materials and instruments needed to complete the surgical procedure.
Surgical Phase
Connective Tissue
Connective tissue needs to be harvested from the patient to help augment the missing soft tissue at the surgical site or a tissue bank allograft needs to be procured before surgery.
When connective tissue will be harvested, the donor tissue is taken from the patient’s palate. The initial incision should be made perpendicular to the long axis of the molars 5 mm from the gingival margin to ensure that the tissue coronal to the incision is over bone and adequate blood supply is maintained to the gingival marginal tissue. This incision should be made to the bone beginning at the mesial of the first molar (to avoid the greater palatine vessels) and may be extended to the mesial of the canine. A second incision is made 2 mm to 3 mm apical to the initial incision and should contact the bone approximately 10 mm to 15 mm apically toward the midpalatal suture creating a partial thickness incision.2 Care should be taken to avoid vasculature running anteriorly from the greater palatine foramen toward the canine area. These vessels run in a groove typically found midway between the midpalatal suture and the crestal bone. Vertical releasing incisions should be avoided on the palate to prevent excision of these vessels.
A periosteal elevator should be introduced into the first incision and the connective tissue elevated to remove the tissue between the epithelium and palatal bone. The wedge of tissue is removed by grasping the band of epithelial tissue and teasing out the attached connective tissue. Wet gauze should be placed over the donor site and firm pressure exerted for 5 minutes to prevent hematoma formation under the epithelial tissue. The incision should then be closed with resorbable sutures in a crisscross-style horizontal mattress suture.3 Patient comfort may be increased by use of a vacuform stent worn for the first 2 weeks after harvesting of the palatal graft.4
After harvesting of the palatal connective tissue, a scalpel is used to remove any adipose tissue and adjust the thickness of the graft. The tissue should be placed in gauze soaked in physiologic saline while preparing the recipient site.
Acellular Dermal Allograft
If an acellular dermal allograft is to be used in place of connective tissue derived from the patient’s palate, reconstitution of the tissue will be necessary. The piece of allograft should be removed from its sterile packaging and placed into a sterile dish containing 50 mL of sterile saline and allowed to soak for 5 minutes. The allograft should then be transferred to a second sterile dish with 50 mL of fresh sterile saline for 5 minutes. At the completion of the second soak, the allograft should be transferred to a separate sterile dish containing 250 mg of tetracycline dissolved into 50 mL of sterile saline and allowed to soak for a minimum of 15 minutes before surgical placement. Several pieces of 2 x 2 gauze should be soaked in a solution of 250 mg of tetracycline and 50 mL of sterile saline in a separate container to be used at the completion of the surgery.
The benefit of using acellular dermal tissue compared to a bioresorbable bovine collagen membrane is the acellular graft contains the blood vessel channels that were present before harvesting the tissue. This permits the recipient site to use the allograft as a scaffold, connecting existing vessels to these open channels and establishing vascularization more rapidly then could be achieved with bovine collagen membranes.
Palatal Donor Tissue vs Acellular Dermal Allograft
There are pros and cons to both palatal donor tissue and acellular dermal allograft for connective tissue. Patient-derived connective tissue has no additional cost added to the procedure, but there is a higher morbidity then when an acellular graft is chosen. Patient comfort after the procedure is also a factor because graft acquisition from the palate can result in discomfort during the healing phase.
By contrast, although there is a higher cost involved with an acellular graft, patient comfort after surgery is higher because no surgery is performed on the hard palate. Additionally, the amount of tissue required is not influenced by what is available on the patient. With regard to the rate of healing, palatal donor tissue will heal quicker then allograft.5 However, studies published comparing the two sources of connective tissue have not found any clinical differences when used in dental applications.6,7 These results mimick what has been seen dermatologically.8
There is one caution that should be considered when considering allograft. Because the graft is processed with tetracycline, the use of this material should be avoided in patients with a known sensitivity to tetracycline drugs. In these patients, use of palatal tissue may be a more prudent option.
Preparation of the Graft Site
Surface bacterial levels intraorally can be reduced by having the patient rinse with an approved rinse such as chlorhexidine, Listerine® (Pfizer, Inc, Morris Plains, NJ), Chloraseptic® (Prestige Brands, Inc, Irvington, NY), or such rinse for another 30 seconds before the initiation of surgery.
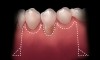
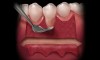
A local anesthetic should be administered mesially and distally of the intended surgical site. A sulcular incision is then started one tooth distal to the tooth/teeth to be treated. The incision is continued to the opposing side one tooth from the site requiring treatment. The papilla should be spared and left attached to the lingual soft tissue to help eliminate soft tissue loss interproximally. Vertical releasing incisions are next made bilaterally and carried beyond the mucogingival junction, ending in Burrow’s triangles (Figure 1). A Burrow’s triangle is used to relieve tension at the most apical extent of a vertical releasing incision to improve flap mobility and achieve tension-free closure.9
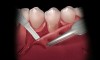
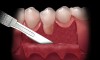
The Burrow’s triangle is then grasped by a tissue forceps and a split thickness dissection with a 15C blade is accomplished to remove this tissue (Figure 2).10 Using a 15C scalpel blade and holding the flap margins with tissue forceps, a split thickness flap is reflected past the mucogingival junction (Figure 3).
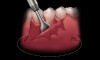
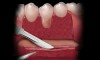
Coronal to the Burrow’s triangles using a new 15C blade, an incision should be carried across the apical extent of the split thickness bed down to the underlying bone (Figure 4). Using a periosteal elevator, a full thickness flap should be reflected from the horizontal incision to a level approximately 5 mm to 10 mm apical to the Burrow’s triangles (Figure 5). Undermining of the tissue bilaterally will aid in flap placement and closure.
Using hand instrumentation, the tooth is scaled and root planed, performing odontoplasty as needed, to reduce root surface convexity (Figure 6). The key is to plane the extruding root surface so that it lies within the buccal plate of bone and does not protrude buccally. Care should be taken not to excessively plane because this may encroach on the root canal system leading to the need for additional intervention.11
Using a new 15C blade, and holding the flap with tissue forceps, the periosteum should be scored, taking care not to perforate the flap (Figure 7). This incision will help mobilize the flap, allowing it to be “stretched” to a more coronal position with no tension in the flap.
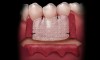
If acelluar connective tissue is used, place the rehydrated piece of acellular dermal allograft on the site to allow blood to contact the material, making sure to touch both sides to the recipient bed. The side that retains the coloration from the blood (red side) should be placed over the recipient bed facing away from the roots. This technique places the “white” side (basement membrane side) toward the root surface to be grafted. The connective tissue is placed over the root exposure and secured with 5-0 polyglycolic acid (PGA) sutures (Figure 8).12 The apical boarder of the graft is not sutured so that apical tension is not placed upon the graft during function as healing occurs.13
The flap should be coronally positioned over the graft and secured with 4-0 PGA (3/8 circle reverse cutting needle) (Figure 9). Using a sling suturing technique, the coronal flap margin is fixated to the lingual soft tissue.14,15 Releasing incisions and Burrow’s triangles are secured with 5-0 PGA (1/2 circle reverse cutting needle). Interrupted sutures are then used to secure the lateral graft borders. It is not critical that all of the graft is covered, and having some portion of the graft exposed at the gingival margin will not affect healing.
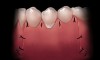
Wet gauze should be placed over the graft site and finger pressure exerted for 5 minutes to prevent hematoma formation under the flap and graft (Figure 10). This will also allow the fibrin in the site to act as a tissue glue temporarily “tacking” the tissues together. Triple antibiotic ointment should be applied over the site and a periodontal dressing mixed and placed to protect the surgical site during the first few days after the procedure (Figure 11).
Postoperative Care
The patient should be instructed to avoid brushing the area for the first 3 weeks after surgery. Rinsing with an over-the-counter antiseptic mouthwash will help maintain hygiene. Warm salt water rinses may also be used. Granular or crunchy foods should be avoided during this time because particles may migrate under the flap margins and irritate the site.
The periodontal dressing can be removed after 7 days. The sutures should be allowed to resorb on their own or be left in place for 21 days. Early suture removal may result in apical positioning of the gingival margin, compromising the desired results.
Case Examples
Case One
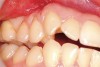
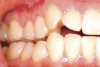
This patient presented with moderate gingival recession and minimal existing attached keratinized tissue (Figure 12). The patient’s chief complaint was discomfort when brushing. A subepithelial connective tissue allograft was placed to create a zone of attached connective tissue and achieve considerable coverage of the previously exposed root surfaces (Figure 13).
Case Two
The patient presented with gingival recession and a complaint of cold sensitivity in the maxillary first and second bicuspid teeth (Figure 14). Minimal attached gingiva was noted. A connective tissue graft was placed to widen the band of attached gingiva and eliminate the cold sensitivity present presurgically (Figure 15).
Conclusion
Achieving root coverage in treating gingival recession has become a more predictable treatment modality. Case selection is important. Surgical intervention has been shown to yield a more predictable long-term result with more complete coverage when treatment is initiated before complete loss of the attached gingiva at the tooth demonstrating recession. The acellular dermal graft has has shown identical long-term results as previously reported with palatal connective tissue grafts. These results suggest that acellular dermal grafts may be a useful substitute for autogenous connective tissue grafts in root coverage procedures.16
References
1. Rose L. Surgical therapies for the treatment of gingival recession. Inside Dentistry. 2006;2(4):66-70.
2. Sato N. Periodontal Suturing, A Clinical Atlas. Chicago: Quintessence Publishing Co; 2000:134-135.
3. Silverstein LH. Dental Principles of Suturing. Mahwah, NJ: Montage Publishing; 2000.
4. Wolf HF, Rateitschak-Pluss EM, Rateitschak KH. Color Atlas of Dental Medicine. Periodontology. New York: Thieme Medical Publishers; 1989:298.
5. Tal H, Moses O, Zohar R, et al. Root coverage of advanced gingival recession: a comparative study between acellular dermal matrix allograft and subepithelial connective tissue grafts. J Periodontol. 2002;73(12):1405-1411.
6. Santos A, Goumenos G, Pascual A. Management of gingival recession by the use of a acellular dermal graft material: a 12-case series. J Periodontal. 2005;76(11):1982-1990.
7. Novaes AB Jr, Grisi DC, Molina GO, et al. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001;
72(11):1477-1484.
8. Li TG, Shorr N, Goldberg RA. Comparison of the efficacy of hard palate grafts with acellular human dermis grafts in lower eyelid surgery. Plast Reconstr Surg. 2005;116(3):873-878.
9. Silverstein LH. Practical procedures: connective tissue grafting using alloderm. Pract Proced Aesthet Dent. 2004;16(10):1-4.
10. Silverstein LH, Shatz PC. Instrumentation for modern day implant surgery. Pract Proced Aesthet Dent. 2005;9.
11. Silverstein LH. Anatomic principles for gingival recontouring. Pract Proced Aesthet Dent. 2003;15(10):13.
12. Silverstein LH. Suturing principles. Applied techniques for predictable suture placement. Part I. Pract Proced Aesthet Dent. 2002;14(3):229-231.
13. Silverstein LH. Essential principles of dental suturing for the implant surgeon. Dent Implantol Update. 2005;16(1):1-7.
14. Silverstein LH, Kurtzman GM. A review of dental suturing for optimal soft-tissue management. Compend Contin Educ Dent. 2005;26(3):163-170.
15. Kurtzman GM, Silverstein LH, Shatz PC, et al. Suturing for surgical success. Dent Today. 2005;24(10):96-102.
16. Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72(8):998-1005.
About the Authors
Gregori M. Kurtzman, DDS
Private Practice
Silver Spring, Maryland
Lee H. Silverstein, DDS, MS
Associate Clinical Professor of Periodontics
Medical College of Georgia
Augusta, Georgia
Private Practice,
Marietta, Georgia
David Kurtzman, DDS
General Private Practice
Hospital-Based Practice Treating Special
Needs Patients
Marietta, Georgia
Peter C. Shatz, DDS
Assistant Clinical Professor of Periodontics
Medical College of Georgia
Augusta, Georgia
Private Practice,
Marietta, Georgia