You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
As clinicians, we must follow developments as new drugs and new drug combinations are introduced to clinical practice. At the same time, there are a number of basic pharmacological properties that are important to understating how local anesthetic drugs work. This is true whether using them individually or in combination for safe and effective pain control.
Dental Local Anesthetics-The Amides
All five North American injectable local anesthetic agents in dental cartridges are amides. Compared to pre-amide injectable ester drugs (which are metabolized in the blood by esterases) amides are metabolized more slowly, primarily in the liver by the cytochrome (CYP450) or P450 isoenzyme system (see Table 1: Common Dental Amide Local Anesthetic Formulations).
Shared characteristics include reasonably low systemic toxicity and allergy potential, adequate onsets and durations of action, and relatively rapid biotransformation. All are minimally irritating and have the potential to damage nerve tissues. Only two (lidocaine and prilocaine) are available as both topical and injectable agents in dentistry.
Basic Pharmacological Actions and Properties of Local Anesthetic Drugs
The two pharmacological actions of drugs in the body are referred to as pharmacodynamics and pharmacokinetics. A major difference between local anesthetic drugs and most other drugs is that their desired effects are local, and systemic effects are undesirable. Patient-centered planning must take into consideration the health status of systems and tissues that may be exposed to the selected LA agents.
The pharmacodynamics of local anesthetic drugs include their effects on peripheral nerves, the central nervous system (CNS), cardiovascular system (CVS), and other tissues, as well as the mechanisms of those actions. The primary reason for administering local anesthetics is to interrupt the normal generation and conduction of nerve impulses. Local anesthetic drugs combined with vasoconstrictors exert multiple different effects; for example, a depressant effect on the CNS (by the LA drug) and a stimulant effect on the CVS (by the vasoconstrictor).
Pharmacokinetics refers to the manner in which the body manages a drug, specifically the mechanisms of absorption, distribution, metabolism (biotransformation), and elimination.
It is important to keep in mind that pharmacology is far from a static discipline.
The five properties that will be discussed here include relative potency, toxicity, vasoactivity, and plasma protein binding capacity, as well as elimination half-life.
RELATIVE POTENCY
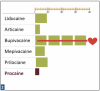
Relative Potency is the relationship between the desired therapeutic effect of a drug and the dose necessary to achieve that effect. Potency is reflected as the concentration (percentage) of the drug per mL of solution. Figure 1: Relative Potency of Dental LA Drugs shows the relative potency comparison for the five commonly used drugs.
Clinical Relevance
Relative potency refers to the amount of a particular drug required to produce the same therapeutic effect as other drugs, in this case other injectable local anesthetics. More potent LA drugs are effective at lower dosages. This discussion hinges on whether the comparison is between actual milligrams of one drug and milligrams of another drug, or between formulations of drugs available by cartridge. Comparing lidocaine and articaine is a common example of comparing potency. Each milligram of lidocaine is more potent than each milligram of articaine; however, each cartridge of 2% lidocaine is less potent than each cartridge of 4% articaine, which has been reported in many applications (for example pulpitis, mandibular infiltration, and palatal anesthesia resulting from buccal infiltration).1,2,3
RELATIVE TOXICITY
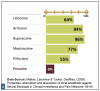
Relative toxicity refers to the relationship of a drug's therapeutic dose to the potential for toxic effects. Relative toxicity for the commonly used dental LA drugs is reflected as a per-milligram comparison in Figure 2: Relative Toxicity of Dental LA Drugs. For example, when comparing lidocaine to articaine, milligram to milligram articaine is 40% less toxic than lidocaine.
When adjusted for relative potency per cartridge, a drug with a lower toxicity per milligram may have a higher toxicity per cartridge than another drug. For example, when compared by cartridge volumes, one cartridge of 4% articaine (72 mg drug) is somewhat more toxic than one cartridge of 2% lidocaine (36 mg drug).
Bupivacaine's significantly greater toxicity is due to its early myocardial depressant effects in addition to its profound (approximately four times greater) CNS depressant effects. In the event of local anesthetic systemic toxicity (LAST) with bupivacaine, myocardial depression signs and symptoms may precede those of CNS depression.
Clinical Relevance
Relative toxicity is considered in a similar but opposite manner to potency. In the lidocaine-articaine example, lidocaine is formulated as a 2% drug to balance effectiveness with toxicity. Comparing milligram to milligram, articaine is less toxic than lidocaine (about 40% more toxic).4,5Therefore, comparing mg to mg, one-half cartridge of 4% articaine (36 mg) is less toxic than one cartridge of 2% lidocaine (36 mg). On the other hand, one full cartridge of 4% articaine (72 mg) is more toxic than one full cartridge of 2% lidocaine (36 mg) but not twice as toxic, despite containing twice as many milligrams (based on 1.8 mL). Articaine's lower toxicity is partly due to its carboxyesterase metabolism, which allows it to be eliminated much more rapidly. Articaine's primarily non-hepatic metabolic pathway decreases its impact on the liver, making it a safer alternative in liver compromise, depending on the degree of compromise. All local anesthetics are capable of causing LAST. As with all local anesthetic drugs, the least amount that results in desired anesthesia should be administered.
Vasoactivity
The vasoactivity of local anesthetic drugs (without the addition of vasoconstrictors) refers to their actions on peripheral vessels. Most local anesthetic drugs are peripheral vasodilators. This property promotes systemic absorption before the anesthetic reaches nerve membranes, limiting effective anesthesia and increasing the risk for systemic toxicity. Countering this effect is one of the reasons vasoconstrictors are added to most dental LA formulations. The addition of vasoconstrictors to vasodilating LA drugs decreases the risk for toxicity and increases the durable effectiveness of the drugs (see Figure 3: Vasoactivity of Dental LA Drugs).
When considering the vasoactivity of LA drugs, another clinically useful perspective is the benefit of weak vasoactivity; for example, 3% mepivacaine plain and 4% prilocaine plain often provide useful durations in the absence of vasoconstrictors.
Clinical Relevance
Vasoactivity relates to the degree to which vasoconstriction is required to counteract the vasodilative nature of local anesthetics. Vasoactivity influences both the safety and duration of anesthesia. Two of these drugs, mepivacaine and prilocaine, are weak enough vasodilators to be clinically useful without vasoconstriction. Clinicians should be aware that plain solutions of these drugs are potentially more toxic because they are absorbed into circulation more rapidly than formulations with vasoconstrictors.
Some drugs, while nearly equal in vasodilatory effects, will work well with lower concentrations of vasoconstrictors (such as 1:200,000 epi) because they are more potent, more diffusible, and/or have a higher plasma protein binding capacity (such as 0.5% bupivacaine and 4% articaine).
Plasma Protein Binding
Plasma protein binding refers to the degree to which drugs attach to proteins in the blood. Bound drugs are too large to pass through biologic membranes; only free drugs are available for delivery to the tissues and to produce the desired pharmacologic action. Therefore, the degree of protein binding can greatly affect the pharmacology of drugs deposited into or subsequently reaching the circulation. Local anesthetics, on the other hand, are deposited into the tissues. Once there, they benefit from similar protein binding strengths in sodium ion channels.6
Bupivacaine and articaine, for example, have strong binding strengths, while prilocaine and procaine have significantly weaker binding strengths (see Figure 4: Plasma Protein Binding Strength of Dental LA Drugs).
Clinical Relevance
The plasma binding capacity of LA drugs refers to the strength of their bonds to receptor proteins in sodium ion channels. Greater receptor protein binding increases the duration of local anesthetics. The more highly protein-bound the LA drug, the longer its duration of action. Those with stronger bonds in the channels will maintain closed positions longer, inhibiting the generation of impulses for longer periods of time.
Elimination Half-Life (t1/2)
Each elimination half-life t1/2 refers to the time it takes for a drug to be reduced from its current concentration to half the current concentration in circulation. See Figure 5: Elimination Half-Life, for estimates for dental local anesthetic drugs. For example, when comparing articaine to lidocaine, the half-life of articaine is approximately 15-45 minutes7,8(depending on volume administered) while the half-life of lidocaine is approximately 90 minutes (see Figure 6: Contrast Elimination Half-Life of Articaine vs Lidocaine). This process continues until all drug has been eliminated from circulation.
Clinical Relevance
Elimination half-life is useful when approaching MRDs to determine appropriate intervals for additional administrations of LA drugs or when considering scheduling multiple appointments on the same day. Understanding t1/2 can be especially valuable when planning procedures with patients who are nursing. The drug with the shortest elimination half-life is articaine. Product Instructions for Use state: "When using [articaine], nursing mothers may choose to pump and discard breast milk for approximately 4 hours (based on plasma half-life) following an injection of [articaine] (to minimize infant ingestion) and then resume breastfeeding."8
pH OF LA WITH VASOCONSTRICTORS
Vasoconstrictors are added to most dental LA formulations to counter the vasoactive effects of LA drugs. While they increase anesthesia durations and reduce risks for systemic toxicity, they also greatly lower the pH of LA solutions. This in turn lowers the number of neutral base molecules available to cross nerve membranes and establish anesthesia, which results in longer onset times. For pH ranges of local anesthetics in dental cartridges, with and without vasoconstrictors, see Figure 7: pH of Dental LA Drugs.
Clinical Relevance of Buffering Local Anesthetics9,10,11,12
Every time LA drugs are administered, innate (natural) carbonate buffering occurs, increasing the pH of the tissues and quickly counteracting the effects of the acidity of LA drugs (see Figure 7: pH of Dental LA Drugs). This type of buffering is responsible for successful LA procedures even with a range of pH among drugs. While onset times are reduced, buffering with sodium bicarbonate solutions just prior to injection provides significantly greater benefits.
Buffering immediately before administration of local anesthetics modifies the equilibrium of the two forms of local anesthetic molecules in solution, decreasing charged cation molecule concentrations and increasing neutral base molecule concentrations. Studies have demonstrated that mixing buffering agents with LA drugs immediately before injection greatly enhances LA drug efficacy by way of CO2 generated when mixing these drugs with sodium bicarbonate.
Carbon dioxide molecules, like neutral base molecules, have a neutral charge distribution and readily penetrate nerve membranes which respond rapidly to CO2's temporary inhibition of depolarization, i.e., blocking impulse generation. This is a recognized effect of CO2, enabling faster onsets of action and decreased pain perception. In a buffered solution, more LA molecules penetrate nerve membranes faster, theoretically to quickly replace the temporary numbing effects of CO2.10 This has been described as a direct depressant or independent anesthetic effect on nerves.11,12 In addition to an independent depressant effect, Catchlove proposed two other advantages of buffering.13 In addition to providing a rapid onset and more comfortable injection, CO2 entering the axoplasm rapidly acidifies the immediate environment by combining with hydrogen ions to form carbonic acid. Reduced axoplasmic pH assures an abundant supply of cationic LA molecules which are responsible for binding sodium ion channels. The excesses of cations produced in this manner, similar to the cations injected into areas surrounding nerve membranes, cannot pass through the nerve membrane. Curatolo, et al. noted that they remain trapped in the axoplasm, increasing the depth of anesthesia.14 This may increase the maximum numbers of sodium ion channels that are exposed to cations, ramping up the inhibition of the generation and conduction of impulses to the CNS. While mechanisms remain speculative, buffering has been demonstrated to increase local anesthetic success significantly.15
Not only does efficacy increase significantly with buffering, but depositing less solution is recommended compared to non-buffered depositions for the same technique.16 Sodium bicarbonate buffering solutions, by virtue of being diluted with buffering agents (LA drug mixed with specific volumes of buffering solutions), are less concentrated. In effect, buffered LA drugs are slightly less concentrated and have been demonstrated to be more effective than their non-buffered counterparts even when somewhat less concentrated. A recent systematic review of the effectiveness of buffered drugs in pulpitis of the mandible pointed to a 2.29 times overall greater efficacy compared to non-buffered, slightly more concentrated counterparts.15See some examples of buffered versus non-buffered drug pH in Figure 8.
Technology is working toward simplifying the process of chairside buffering; for example, bifurcated cartridges have been proposed with acceptable shelf lives as well as the ability to buffer immediately prior to administration, which is essential to enhanced effects of CO2 in the process.
2015 Update on FDA Drug Labeling for Pregnancy and Lactation17
Planning safe individualized care for pregnant and lactating patients requires up-to-date knowledge of current FDA guidance on drug precautions and contraindication. To reduce risks associated with drugs administered for dental treatment, dental hygienists share in the responsibility to identify and discuss these implications with patients as they relate to planned treatment, for example, the appropriate integration of local anesthetics into patient care. To do this, dental healthcare providers have referenced the long standing "FDA Pregnancy and Lactation Drug Categories" in use since 1979, however this reference no longer exists.
In 2015, the FDA implemented a change to the long-standing system for rating drug risks during pregnancy and lactation. In an effort to provide product information that is more meaningful to both patients and healthcare providers, the widely recognized system of letter-based risk categories (A, B, C, D, and X) on prescription and biological drug labels was replaced with a new information format. This change was made to address concerns that the old letter system resulted in false assumptions about the actual meaning of the letters and left patients and providers ill-informed.
The new labeling system is designed to facilitate better patient-centered counseling and informed decision making for pregnant individuals considering drugs recommended for their healthcare needs (see Figure 9). Although the new labeling format does guide improved individualized care decisions, it still does not provide a definitive "yes" or "no" answer in most cases. Clinical interpretation is still required on a case-by-case basis. For example, selecting local anesthetics is not as simple as one of two favored categories-B or C. Each drug should be considered based on its unique pharmacological properties, pharmacokinetics, and the specific patient history.
While the new Pregnancy and Lactation Labeling Final Rule (PLLR) went into effect on June 30, 2015, the implementation timeline of changes in product directions for use, including drug labels and package inserts, is variable (see Table 2). The A, B, C, D, and X risk categories, in use since 1979, are now replaced with narrative presentations of information related to use of a drug during pregnancy and lactation including: Risk Summary, Clinical Considerations for Use, and Supporting Data. A lactation subsection provides information about using the drug while breastfeeding, including, the amount of drug in breast milk and potential effects on the breastfed infant, and a subsection on females and males of reproductive potential with information about the need for (1) pregnancy testing, (2) contraception, and (3) infertility.
Considering the example of dental local anesthetics, all anesthetics currently available in dental cartridges were FDA approved prior to 2001. These companies will not be required to include the new PLLR risk sections, but they are required to remove reference to the A, B, C, D, and X risk categories. See Table 2 for the FDA timeline for produce label changes.
CONCLUSION
The five injectable local anesthetic agents used in dental cartridges are all amides (articaine, bupivacaine, lidocaine, mepivacaine, and prilocaine). Several pharmacological properties are essential to comprehending how local anesthetic drugs work. Incorporating their pharmacological properties into patient-centered dental pain management, whether using LA drugs individually or in combination, enhances their safe and effective use. In addition, buffering has been demonstrated to significantly enhance local anesthetic success.
References
1. de Geus JL, Noguiera da Costa JK, Maran BM, Loguercio AD, Reis A. Different anesthetics on the efficacy of inferior alveolar nerve block in patients with irreversible pulpitis, J Am Dent Assoc, 2020; 151(2): 87-97.
2. Al-Mahalawy H, Abuohashish H, Chathoth S, Al-Masoud N, Al-Jandan B. Articaine versus lidocaine concentration in palatal tissues after supraperiosteal buccal infiltration anesthesia, J Oral and Max Surg. 2018; 76(2): 315.e1-135, e7.
3. Rathi NV, Khati AA, Agrawal AG, Baliga M, Thosar NR, Deolia SG. Anesthetic efficacy of buccal infiltration articaine versus lidocaine for extraction of primary molar teeth, Anes Prog. 2019; 66(1):3-7
4. Scully C. Scully's Medical Problems in Dentistry. 7th ed. London: Churchhill Livingstone; 2014:51-96.
5. Jastak JT, Yagiela JA, Donaldson D. Local Anesthesia of the Oral Cavity. Philadelphia: WB Saunders; 1995.
6. (Snoeck M. Articaine: a review of its use for local and regional anesthesia. Local Reg Anesth. 2012;5:23-33. doi:10.2147/LRA.S16682
7. Oertel R, Ebert U, Rahn R, Kirch W. The effect of age on pharmacokinetics of the local anesthetic drug articaine. Reg Anesth Pain Med. 1999;24(6):524-528.
8. SEPTODONT (2019). Articaine hydrochloride and epinephrine injection. Instructions for Use, Rev, 10/2019 (2552-6). SEPTODONT USA, Lancaster, PA; www.septodontusa.com. Accessed July 24, 2022.
9. Mather, Laurence & Tucker, Geoffrey. (2009). Properties, absorption and disposition of local anesthetic agents. Neural Blockade in Clinical Anesthesia and Pain Medicine. 48-95.
10. Donaldson M, Goodchild JH. Taking local anesthesia to the next level: Four strategies clinicians may consider. Compend Contin Educ Dent. 2022;43:20-24
11. Donaldson M, Goodchild JH. Comparing the pH change of local anesthetic solutions using two chairside buffering techniques. Compend Contin Educ Dent. 2016;37:e6-e12.
12. Malamed SF, Tavana S, Falkel M. Faster onset and more comfortable injection with alkalinized 2% lidocaine with epinephrine 1:100,000. Compend Contin Educ Dent. 2013;34:10-20.
13. Catchlove RFH. The influence of CO2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972;181(2):
298-309.
14. Curatolo M, Petersen-Felix S, Arendt-Nielsen L. et al. Adding sodium bicarbonate to lidocaine enhances the depth of epidural blockade. Anesth Analg. 1988;86(2):341-347.
15. Kattan S, Lee S-M, Hersh EV, Karabucak B. Do buffered local anesthetics provide more successful anesthesia over non-buffered solutions in patients requiring dental therapy? - A systematic review and meta-analysis. Jour Am Dent Assn. 2019;150(3):165-177.
16. DiMarco AC, Bassett KB. Perspectives on buffered articaine in dentistry, using buffered articaine offers several clinical advantages in dental pain management. Decision in Dentisty. June 7, 2022. Accessed July 24, 2020.
17. Reference: Pregnancy and Lactation Labeling (Drugs) Final Rule | FDA. Available at: https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule. Accessed May 1, 2022.