You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Dense polytetrafluoroethylene (d-PTFE) is a nonresorbable material that can be used as a cell occlusive barrier when performing guided bone (GBR) or guided tissue (GTR) regeneration procedures.1 GBR is often employed to restore defective alveolar ridges post extractions prior to or in conjunction with dental implant insertion.2 This technique usually involves inserting a bone graft and covering it with a barrier, which is either resorbable or nonresorbable. A barrier is used to prevent epithelium and connective tissue from migrating into the grafted site, thereby facilitating repopulation of the bone graft with progenitor cells from the adjacent bone.3
d-PTFE utilization avoids potential problems encountered with other types of barriers used during GBR procedures. For example, both resorbable and nonresorbable barriers (eg, expanded polytetrafluoroethylene [e-PTFE]) need to be completely submerged at the time of placement, because if they are left or become exposed, there may be negative consequences. If a resorbable barrier is not submerged or becomes exposed to oral fluids, it will not be fully intact in 7 days and will usually resorb quickly (approximately 2 weeks).4 This will not provide enough time for progenitor cells to repopulate the graft, and the progenitor cells will not have sufficient time to differentiate and create osteoid tissue (connective tissue that is the precursor of bone). Conversely, when a nonresorbable barrier—such as e-PTFE—is employed and becomes exposed due to a soft-tissue dehiscence, it is susceptible to becoming infected within 4 weeks.5,6 These negative issues can be avoided if d-PTFE barriers are used. They do not have to be submerged, and they will not resorb prematurely. Furthermore, surgical sites treated with d-PTFE barriers do not become infected when they remain uncovered for 4 to 6 weeks.7-9 d-PTFE barriers are marketed under various names by several companies (eg, Cytoplast™ [Osteogenics Biomedical, www.osteogenics.com], TefGen FD™ [Lifecore Biomedical, LLC, www.lifecore.com], Symbios OsteoShield® [DENTSPLY Implants, www.dentsplyimplants.us]). This article addresses the use of d-PTFE (Cytoplast) in GBR procedures and discusses the benefits of these barriers as well as data limitations. Since the larger studies in the literature addressing d-PTFE barriers have employed Cytoplast7-10 and the authors’ cases were performed with this type of barrier, this article will focus on this brand of d-PTFE.
Historical Perspective
In 1976, Melcher11 discussed the potential of the periodontal ligament (PDL) to provide progenitor cells to enhance regeneration of osseous defects. Subsequently, Nyman et al12 used a Millipore filter (barrier) to exclude epithelium and connective tissue from human periodontal defects to help repopulate osseous defects with progenitor cells from the PDL. This was called guided tissue regeneration (GTR) and its goal was to regenerate bone, PDL, and cementum. Subsequently, the same principles were used by Dahlin et al in a rabbit model to augment a ridge, and this was labeled GBR.13
The main GBR principles are as follows: 1) exclude epithelium and connective tissue to allow progenitor cells to repopulate the treated site; 2) create a space under the barrier, which will fill in with bone (space maintenance)—the barrier is often held up with a bone graft or tenting screws; 3) the barrier protects the clot—this is referred to as “clot stabilization”; 4) attain angiogenesis; and 5) achieve primary closure by flap advancement.14
Primary closure often requires periosteal and vertical releasing incisions to facilitate flap advancement.15 If a periosteal releasing incision does not provide adequate flap advancement, then the tissue may need to be incised more deeply into the submucosa to attain primary closure.15 This will probably increase a patient’s morbidity (edema, ecchymosis, and discomfort). Thus, avoidance of this step would be advantageous if equivalent regenerative results could be attained without primary closure.
Characteristics of d-PTFE Barriers
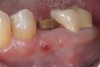
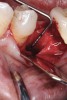
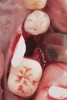
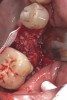
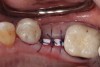
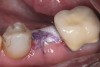
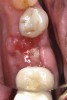
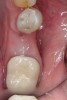
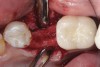
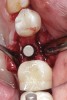
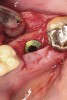
Nonresorbable barriers (e-PTFE) first appeared as Gore-Tex®, and then TefGen became commercially available.1 TefGen was considered a nano-porous barrier (n-PTFE), a term denoting that pores were extremely small.16 Characteristics of Cytoplast barriers, which were used in the four cases illustrated in this article (Figure 1 through Figure 25), are described as follows: The d-PTFE barriers have a 4-year shelf life.1 Cytoplast Regentex™ is textured on one side (Figure 3). The texture consists of small hexagonal dimples, which increase the barrier’s surface area by 250%.17 When inserting the barrier, the shiny, undimpled side is placed down onto the bone graft so that the dimpled side comes into contact with the overlying flap (Figure 6). Under the flap, fibroblasts migrate into the shallow dimples; this provides a grip for the soft tissue (adhesion). Available barrier sizes are 12 mm x 20 mm and 25 mm x 30 mm. The d-PTFE barrier is 0.13-mm to 0.25-mm thick. Other types of this brand of barriers are available (eg, titanium reinforced).
Barriers: d-PTFE vs e-PTFE
Barriers used for GBR procedures are referred to by a variety of names, including: membranes; barrier membranes; biologic barriers; occlusive membranes; bioresorbable (which need to be degraded by the body), bioabsorbable (which dissolve in body fluids without polymer chain cleavage), and biodegradable (which break down due to macromolecular degradation) barriers; and resorbable and nonresorbable barriers.18 Nonresorbable membranes can be divided into two categories: expanded (e-PTFE) and dense (d-PTFE) polytetrafluoroethylene. The material is the same for both barriers, but the e-PTFE has been stretched. The pore size for e-PTFE (trade name Gore-Tex) is 5 to 30 microns (µm), and d-PTFE (Cytoplast) pore size is 0.2 µm.1 With regard to timing of removal of nonresorbable barriers, it has been suggested that they be removed in 4 to 6 weeks for GTR procedures and 6 to 9 months for GBR.18
When the efficacy of d-PFTE was compared to e-PTFE to achieve GTR in osseous defects, the studies demonstrated there were no statistically significant differences with respect to gaining bone.19-21 In these three clinical trials the barriers were submerged, and alloplastic bone20,21 or a xenograft was used.19 Barriers were left in place 6 to 8 weeks. No studies compared the regenerative efficacy of submerged versus exposed barriers.
Infections
The diameter of pathogenic bacteria is generally less than 10 µm.22 Stretching of e-PTFE increases its pore size (5 µm to 30 µm), which allows bacteria to penetrate the barrier. In contrast, d-PTFE (pore size 0.2 µm) is impervious to bacterial penetration. When d-PTFE barriers were experimentally challenged with Enterococcus faecalis, an organism commonly found intraorally (with a size of 0.5 µm to 1 µm), it was noted that the bacteria could not penetrate the barrier (data on file with Osteogenics).
As indicated, when e-PTFE barriers were used, if they became exposed, they had the potential to become infected by around 4 weeks, which could result in loss of part or all of the bone graft.7-9 On the contrary, numerous articles noted that sites treated with d-PTFE barriers, despite exposure to the oral environment, did not become infected within 4 to 6 weeks.1,7-9,23 No data is available with respect to exposure for longer treatment times.
Primary Closure
To attain bone regeneration when e-PTFE and resorbable barriers are used, primary closure is required. This precipitates two issues: 1) tissue contour usually will be altered as a result of primary closure; and 2) the position of the mucogingival junction is frequently changed.15 Thus, keratinized tissue may be translocated lingually, leaving the buccal aspect with only mucosa. In addition, there may be loss of vestibular depth when flaps are advanced. Pertinently, the size of the flap advancement is often related to the amount of patient morbidity. In summary, with d-PTFE barriers, there is no need for primary closure, the surgical procedure is easy, and a second surgery to remove the barrier is avoided.
Preservation of Keratinized Tissue
If a d-PTFE barrier is placed over a bone graft at an extraction socket, and primary closure is not performed, the linear amount of keratinized tissue will be maintained. When there is mid-buccal recession on a tooth and the tooth is removed, if a d-PTFE barrier is placed over a bone graft, the volume of keratinized tissue will be increased. When an extraction is done and no barrier is placed, the healed socket will develop keratinized gingiva, but its volume would be decreased due to partial collapse of the gingiva into the defect. In this regard, Barber et al23 demonstrated that after maxillary anterior teeth were extracted and a d-PTFE barrier was used over a bone graft, the band of keratinized tissue was increased.
Epithelialization over the reddish colored connective tissue (osteoid) usually takes 14 to 21 days. Epithelial cells from the surfaces adjacent to the wound migrate across the connective tissue. They usually migrate 0.5 mm to 1 mm per day after a 12-hour lag period.24
Time, Cost
When comparing the efficiency of using d-PTFE versus other barriers that must be submerged, the need to perform primary closure when utilizing other barriers will require additional time and, therefore, likely result in an increased cost for procedures.
Wound Healing
When a socket heals, the blood clot is replaced with granulation tissue (at around 96 hours),25 which is subsequently converted to connective tissue (at around 21 to 28 days). The connective tissue, osteoid, is the precursor of bone. Osteoblasts initiate bone formation by secreting osteoid as several specific proteins. Osteoid is composed of fibers and ground substance.26 The main fiber-type is Type I collagen, which accounts for 90% of the osteoid. The ground substance is mostly made up of chondroitin sulfate and osteocalcin.26 The osteoid tissue organizes and mineralizes to become woven bone—it takes approximately 3 months for the socket to be filled with bone. Woven bone is immature bone and, with time, it mineralizes further and is referred to as lamellar bone.27 It takes approximately 4 months to develop lamellar bone, and mineralization continues for a year.
Barrier Removal
The timing of barrier removal is a contentious issue, because the minimum amount of time the barrier needs to be in place to facilitate optimal maturation of the osteoid tissue is unknown. Barriers need to be left in place long enough for the osteoid tissue to become condensed and impervious to invasion by other tissues so that its mineralization and conversion to bone is not disturbed. Initially, some researchers suggested that after placement of e-PTFE barriers, they be left in place for a prolonged period of time (eg, 16 weeks) when doing GBR procedures.28-30 Others suggested 4 to 6 weeks31 or 6 weeks32,33 for GTR procedures.
With regard to Cytoplast d-PTFE barriers, investigators have removed them at various time intervals when they were left exposed after surgery (Table 1)7-10,23,34-41: < 3 weeks10; 3 weeks34,35,37; 3 to 4 weeks8,36,38; 4 weeks7,39,41; 4 to 6 weeks9,23; and 6 weeks.40 All authors reported successful results. However, there are several issues that require discussion. The manufacturer recommends that d-PTFE (Cytoplast) barriers be removed at about 4 weeks. Upon removal, there may be some slight bleeding, which reflects disturbance of the biological adherence between tissue and the d-PTFE membrane. In general, soft tissue will not grow over the membrane. After 4 to 6 weeks, the nonresorbable membrane is easily removed without local anesthesia, because the dense surface of the membrane does not allow deep fibrous ingrowth. If it is desired to leave the barrier in place for 8 weeks, when the barrier is removed it will leave an epithelialized pouch in the tissue, because the epithelium will have invaginated around the margin (observations of GG). To correct this issue, the current authors have used a diamond to remove the surface epithelium, which is around 0.3 mm thick.42 The walls of the pouch are then abraded, creating bleeding points, and the sites are sutured. Healing then occurs uneventfully.
Removal of the d-PTFE at 4 or 4 to 6 weeks does not appear to be detrimental to bone regeneration,7,8,11,23,36 as is shown in the four cases presented (Figure 1 through Figure 25). Conceptually, the osteoid tissue is dense enough to preclude penetration of the epithelium or connective tissue that would inhibit healing. There are several possible explanations as to why barriers can be removed earlier than previously recommended:
• Several studies suggested that early barrier removal leads to poor results when performing a ridge augmentation.29,30 However, this conclusion may be incorrect, because these investigations may have erroneously surmised that early barrier removal was associated with lack of regeneration, when in reality, failures were related to soft-tissue dehiscences and bacterial contamination, which resulted in early barrier removal.
• One study in dogs found early barrier removal of e-PTFE (4 weeks) resulted in poorer results than barriers left in place 16 weeks.28 No clear explanation can be provided as to why these findings are not applicable to d-PTFE, but they are contrary to successful results obtained when barriers are removed at 4 weeks.
• There are no studies that sequentially examined healing at different time points to determine the minimum healing time at which osteoid is mature enough to proceed to mineralization.
• Factors that can affect healing are the relationship between the size of defects and the amount of time needed for maturation of the osteoid tissue, and the time for tissue maturation may be site- and patient-specific.
• The studies presented in this article all regenerated bone within the alveolar bony housing. Vertical augmentation was not attempted. Whether or not it is possible to achieve this with barrier removal after 4 weeks and no primary closure needs to be assessed. There have been no reports with respect to this endeavor.
Systematic Literature Review
Carbonell et al43 recently conducted a literature review concerning the efficacy of high-density PTFE for regenerative purposes. The authors found 24 articles that addressed use of d-PTFE and n-PTFE barriers for regeneration around teeth and implants. These included two in-vitro studies, seven experimental studies, and 15 clinical studies. In general, the clinical studies were small or incomplete. They concluded that at present there is limited data supporting the use of n-PTFE membranes. It was mentioned that there may be some use for d-PTFE in GTR and GBR over immediate implants and fresh extraction sockets. However, just because numerous studies have not been conducted, it does not mean that the barriers are ineffective for certain procedures. For instance, clinicians have demonstrated the efficacy of d-PTFE as a barrier for socket preservations7-10 and GBR to replace lost or defective buccal plates of bone.7-9 In this regard, clinical practice is sometimes ahead of scientific evidence, since oftentimes there is insufficient funding to carry out expensive studies.
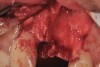
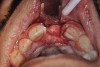
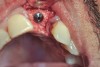
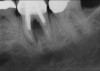
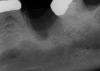
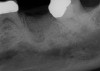
In Figure 1 through Figure 25 the authors present various case reports to demonstrate with radiographs or clinical re-entries that osseous regeneration was achieved with d-PTFE barriers and Puros® bone grafts (Zimmer Dental, www.zimmerdental.com) after extractions. (The technique is discussed after the literature review.)
Data Pertaining to d-PTFE Utilization
Socket preservation: four walls present—Several authors assessed socket preservation using d-PTFE without primary closure. Fotek et al10 evaluated Cytoplast barriers (N = 20) as a method to enhance socket preservation. After extractions, demineralized freeze-dried bone allograft (DFDBA) was placed into the sockets. The barriers were secured by elevating a small flap (3 mm on the buccal and lingual), and the barriers were tucked under the flap buccally and lingually without disturbing the mesial or distal papilla. Part of the barrier was left exposed, and barriers were removed after 4 weeks. They reported that the amount of bone loss was 0.3 mm horizontally and 0.25 mm vertically. Hoffman et al7 also evaluated socket preservation. They used barriers without bone grafts. The vertical amount of bone loss was between 0.5 mm and 1 mm; 14% of the sites in the middle of the socket lost 2 mm, because barriers collapsed due to lack of bone support. Yamashita et al9 employed bone grafts plus Emdogain® (Straumann, www.straumann.us) or plasma-rich protein (PRP) under d-PTFE barriers. They reported no bone loss during socket preservations, but noted that the original sockets were overbuilt by around 0.8 mm.
These were good results with respect to reducing horizontal and vertical bone loss after an extraction. In contrast, a recent systematic review reported that 6 months after tooth removal, which included flap elevation and no other therapy, the extraction sockets showed a mean 1.24-mm vertical bone loss (range 0.9 mm to 3.6 mm) and 3.79-mm horizontal bone decrease (range 2.46 mm to 4.56 mm).44 Others who used a nonresorbable45 or a resorbable barrier46 also found vertical bone loss to be around 1 mm and horizontal bone loss to be around 3 mm. When bone and a barrier were used, horizontal bone loss of around 1 mm was reported along with vertical bone gain of 1.3 mm.47 Without flap elevation, typically there is vertical bone loss, but horizontal bone loss is diminished.48,49
GBR when the buccal plate or part of the buccal plate(s) were resorbed—In several studies, d-PTFE barriers were employed and left exposed during treatment of defective buccal bony plates.7-9 Barboza et al8 assessed the healing of 72 patients when a bone graft and a Cytoplast barrier were used to regenerate a resorbed buccal wall of the socket. The sizes of the defects were not reported. Bone grafts (lyophilized bone) were used to prevent collapse of the membrane. The barriers were removed between 21 and 28 days. No data was presented with respect to bone regeneration, but the authors indicated that all sites successfully received an implant.
Hoffman et al used d-PTFE barriers without adjunctive bone grafts at 276 sockets.7 Among those patients, 28 had defects in the buccal plate. In general, the defects were not greater than 50% of the buccal plate of bone. At these sites, there was a mean bone gain of 6 mm (range of 4 mm to 8 mm). There was no control group receiving any therapy, so it is unclear how much better the result was than osseous debridement. Nevertheless, even without bone grafts the data was encouraging with respect to bone regeneration at defective buccal plates.
Yamashita et al9 used d-PTFE to regenerate 86 sockets and 45 defective ridges in 111 patients. At the 45 ridges where the mean osseous defect was 5.5 mm, there was 5 mm of bone regenerated (95.8% fill). In conjunction with the alloplastic bone grafts, they used Emdogain and/or PRP. Therefore, it was impossible to know precisely which factor(s) accounted for the good results.
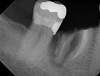
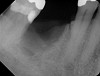
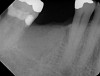
All three studies7-9 addressing defective alveolar ridges reported a positive finding with respect to healing, which facilitated implant placement in all monitored patients. In addition, the four cases presented by the current authors (Figure 1 through Figure 25) attained excellent results with d-PTFE plus an alloplastic bone graft (Puros) when treating buccal wall defects.
Case Presentations
The four presented cases shown in Figure 1 through Figure 25 were all treated in a similar manner as outlined in detail in the captions for the first case (Figure 1 through Figure 11). In all four cases, teeth with pathosis were removed, buccal and lingual flaps were elevated, and sites were surgically debrided. A Cytoplast d-PTFE barrier was sized and inserted usually under the buccal flap, the bone graft was added, the barrier was then tucked under the lingual flap, and the flaps were sutured. Bone grafts were used in all of the cases, because buccal bony plates were resorbed and the bone graft helped support the barrier.
Summary and Conclusions
d-PTFE has many advantages. It is a biocompatible barrier that is as effective as e-PTFE for GTR procedures when they are both submerged and the barrier is left in place 4 to 6 weeks; however, no studies compared the efficacy of these barriers when the d-PTFE was left exposed and the e-PTFE was submerged. d-PTFE barriers are easy to remove. They allow cell adhesion without tissue in-growth into the barriers. The studies that assessed the efficacy of d-PTFE barriers when they were left exposed consistently reported good results with respect to socket preservation. Furthermore, it is advantageous to use a bone graft in conjunction with the d-PTFE to avoid the barrier collapsing into the defect.
There are a limited number of studies assessing the utilization of d-PTFE in conjunction with bone grafts when there are defective alveolar ridges.7-9 However, these papers have consistently demonstrated that bone repair occurs despite the barrier being left exposed. In these reports, all bone repairs appear to be within the housing of the alveolar bone. No studies demonstrated horizontal or vertical augmentation beyond the existing alveolar boundaries. In this regard, it is advisable to overbuild bone-grafted sites, because the authors of this article have attained horizontal augmentation beyond the alveolar housing (Figure 18).
The amount of time that a barrier must be left in place remains controversial. However, it appears that a 4-week interval facilitated developing a dense osteoid tissue, which proceeds to mineralize and form bone with respect to sockets and ridge defects. It may be prudent to leave the barrier in longer for larger defects, but there are no data supporting this concept with d-PTFE barriers. If this is done, it will be necessary to remove the invaginated epithelium with a diamond bur after barrier removal, and the site will need to be sutured.
Despite a total lack of randomized clinical trials, the numerous cases presented demonstrate that this procedure is clinically beneficial with respect to restoring a defective buccal or lingual plate of bone. In conclusion, GBR procedures using d-PTFE are easy to perform and cause less morbidity when compared to procedures that require flap advancement to achieve primary closure.
Addendum: Histological Findings
A recent study by Borg and Mealey50 evaluated the efficacy of socket preservation procedures when d-PTFE was employed in conjunction with a combination mineralized/demineralized allograft versus 100% mineralized FDBA (N = 42). After teeth were removed, a bone graft material was placed and a d-PTFE barrier was inserted as previously described and left exposed. Barriers were removed 4 weeks after placement. The data provided histological evidence that a bone allograft used in conjunction with a d-PTFE barrier resulted in increased vital bone formation. These findings validate that d-PTFE barriers in conjunction with an allograft or combination of allografts result in vital bone formation and help preserve the dimensions of the alveolar ridge. The data also corroborated that 4 weeks was sufficient time for barriers to be effective. Others also have histologically demonstrated that d-PTFE results in vital bone formation. Bartee37 reported vital bone formation after using d-PTFE and a “bone paste,” and Hoffman et al7 indicated that d-PTFE resulted in bone formation; however, bone grafts were not used.
Acknowledgment
The images for Figure 1 through Figure 11 were courtesy of Mehmet Dikmen, DMD, PhD. The images for Figure 12 through Figure 14 were courtesy of Siyan Lin, DDS.
Disclosure
The authors had no disclosures to report.
About the Authors
Gary Greenstein, DDS, MS
Clinical Professor
Department of Periodontology
College of Dental Medicine
Columbia University
Private Practice
Surgical Implantology and Periodontics
Freehold, New York
Joseph R. Carpentieri, DDS
Clinical Assistant Professor
College of Dental Medicine
Columbia University
Private Practice
Surgical Implantology and Prosthodontics
White Plains, New York
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Bartee KB. Implant Site Development and Extraction Site Grafting: Bone Biology & Physiology, Selection of Grafting Materials, Selection of Barrier Membranes and Surgical Technique. Lubbock, TX: Osteogenics Clinical Education; 2012:5-40. https://www.osteogenics.com/media/uploads/resourceDocs/123uqtpgho6u.pdf. Accessed May 23, 2014.
2. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57(1):3-14.
3. Retzepi M, Donos N. Guided Bone Regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010;21(6):567-576.
4. Tal H, Kozlovsky A, Artzi Z, et al. Cross-linked and non-cross-linked collagen barrier membranes disintegrate following surgical exposure to the oral environment: a histological study in the cat. Clin Oral Implants Res. 2008;19(8):760-766.
5. Tempro PJ, Nalbandian J. Colonization of retrieved polytetrafluoroethylene membranes: morphological and microbiological observations. J Periodontol. 1993;64(3):162-168.
6. Selvig KA, Kersten BG, Chamberlain AD, et al. Regenerative surgery of intrabony periodontal defects using ePTFE barrier membranes: scanning electron microscopic evaluation of retrieved membranes versus clinical healing. J Periodontol. 1992;63(12):974-978.
7. Hoffmann O, Bartee BK, Beaumont C, et al. Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: a retrospective non-randomized study. J Periodontol. 2008;79(8):1355-1369.
8. Barboza EP, Stutz B, Ferreira VF, Carvalho W. Guided bone regeneration using nonexpanded polytetrafluoroethylene membranes in preparation for dental implant placements—a report of 420 cases. Implant Dent. 2010;19(1):2-7.
9. Yamashita M, Horita S, Takei N, et al. Minimally invasive alveolar ridge preservation/augmentation procedure. Fukuoka, Japan: Funakoshi Research Institute of Clinical Periodontology. https://www.osteogenics.com/media/uploads/resourceDocs/23ps19cnm12.pdf. Accessed May 14, 2015.
10. Fotek PD, Neiva RF, Wang HL. Comparison of dermal matrix and polytetrafluoroethylene membrane for socket bone augmentation: a clinical and histologic study. J Periodontol. 2009;80(5):776-785.
11. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47(5):256-260.
12. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290-296.
13. Dahlin C, Sennerby L, Lekholm U, et al. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989;4(1):19-25.
14. Bashutski J, Oh TJ, Chan HL, Wang HL. Guided tissue regeneration: a decision-making model. J Int Acad Periodontol. 2011;13(2):48-57.
15. Greenstein G, Greenstein B, Cavallaro J, et al. Flap advancement: practical techniques to attain tension-free primary closure. J Periodontol. 2009;80(1):4-15.
16. Crump TB, Rivera-Hidalgo F, Harrison JW, et al. Influence of three membrane types on healing of bone defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(4):365-374.
17. Cytoplast regeneration products catalog. Osteogenics Biomedical. August 2014. http://www.osstem.de/data/library/2014enCytoplast.pdf. Accessed May 14, 2015.
18. Hutmacher D, Hürzeler MB, Schliephake H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants. 1996;11(5):667-678.
19. Walters SP, Greenwell H, Hill M, et al. Comparison of porous and non-porous teflon membranes plus a xenograft in the treatment of vertical osseous defects: a clinical reentry study. J Periodontol. 2003;74(8):1161-1168.
20. Lee JY, Kim YK, Yun PY, et al. Guided bone regeneration using two types of non-resorbable barrier membranes. J Korean Assoc Oral Maxillofac Surg. 2010;36:275-279.
21. Lamb JW 3rd, Greenwell H, Drisko C, et al. A comparison of porous and non-porous teflon membranes plus demineralized freeze-dried bone allograft in the treatment of class II buccal/lingual furcation defects: a clinical reentry study. J Periodontol. 2001;72(11):1580-1587.
22. Moreno NP, Tharp BZ, Erdmann DB, et al. The Science of Microbes: Comparing Sizes of Microorganisms. Houston, TX: Baylor College of Medicine; 2012. http://www.bioedonline.org/tasks/render/file/index.cfm?fileID=A1289E74-BBD5-07E2-3BC4E155304E18D9. Accessed May 14, 2015.
23. Barber HD, Lignelli J, Smith BM, Bartee BK. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J Oral Maxillofac Surg. 2007;65(4):748-752.
24. Engler WO, Ramfjord SP, Hiniker JJ. Healing following simple gingivectomy. A tritiated thymidine radioautographic study. I. Epithelialization. J Periodontol. 1966;37(4):298-308.
25. Amler MH. Age factor in human alveolar bone repair. J Oral Implantol. 1993;19(2):138-142.
26. Raina V. Normal osteoid tissue. J Clin Pathol. 1972;25(3):229-232.
27. Eriksson C, Ohlson K, Richter K, et al. Callus formation and remodeling at titanium implants. J Biomed Mater Res A. 2007;83(4):1062-1069.
28. Lekholm U, Becker W, Dahlin C, et al. The role of early versus late removal of GTAM membranes on bone formation at oral implants placed into immediate extraction sockets. An experimental study in dogs. Clin Oral Implants Res. 1993:4(3):121-129.
29. Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994:14(2):166-180.
30. Becker W, Becker BE, Handlesman M, et al. Bone formation at dehisced dental implant sites treated with implant augmentation material: a pilot study in dogs. Int J Periodontics Restorative Dent. 1990;10(2):92-101.
31. Tonetti MS, Pini-Prato G, Cortellini P. Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol. 1993;64(10):934-940.
32. Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human infrabony defects. I. Clinical measures. J Periodontol. 1993;64(4):254-260.
33. Caffesse RG, Mota LF, Quiñones CR, Morrison EC. Clinical comparison of resorbable and non-resorbable barriers for guided periodontal tissue regeneration. J Clin Periodontol. 1997;24(10):747-752.
34. Bartee BK. A simplified technique for ridge preservation after tooth extraction. Dent Today. 1995;14(10):62-67.
35. Bartee BK. The use of high-density polytetrafluoroethylene membrane to treat osseous defects: clinical reports. Implant Dent. 1995;4(1):21-26.
36. Bartee BK. A membrane and graft technique for ridge maintenance using high-density polytetrafluoroethylene (n-PTFE) and hydroxylapatite: report of four cases. Tex Dent J. 1995;112(5):7-16.
37. Bartee BK. Evaluation of a new polytetrafluoroethylene guided tissue regeneration membrane in healing extraction sites. Compend Contin Educ Dent. 1998;19(12):1256-1264.
38. Bartee BK. Extraction site reconstruction for alveolar ridge preservation. Part 2: membrane-assisted surgical technique. J Oral Implantol. 2001;27(4):194-197.
39. Zafiropoulos GG, Kasaj A, Hoffmann O. Immediate implant placement in fresh mandibular molar extraction socket: 8-year results. A case report. J Oral Implantol. 2010;36(2):145-151.
40. Waasdorp J, Feldman S. Bone regeneration around immediate implants utilizing a dense polytetrafluoroethylene membrane without primary closure: a report of 3 cases. J Oral Implantol. 2013;39(3):355-361.
41. Yun JH, Jun CM, Oh NS. Secondary closure of an extraction socket using the double-membrane guided bone regeneration technique with immediate implant placement. J Periodontal Implant Sci. 2011;41(5):253-258.
42. Prestin S, Rothschild SI, Betz CS, Kraft M. Measurement of epithelial thickness within the oral cavity using optical coherence tomography. Head Neck. 2012;34(12):1777-1781.
43. Carbonell JM, Martín IS, Santos A, et al. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: a literature review. Int J Oral Maxillofac Surg. 2014;43(1):75-84.
44. Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23(5 suppl):1-21.
45. Lekovic V, Camargo PM, Klokkevold PR, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69(9):1044-1049.
46. Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68(6):563-570.
47. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
48. Degidi M, Daprile G, Nardi D, Piattelli A. Buccal bone plate in immediately placed and restored implant with Bio-Oss® collagen graft: a 1-year follow-up study. Clin Oral Implants Res. 2013;24(11):1201-1205.
49. Brownfield LA, Weltman RL. Ridge preservation with or without an osteoinductive allograft: a clinical, radiographic, micro-computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J Periodontol. 2012;83(5):581-589.
50. Borg TD, Mealey BL. Histologic healing following tooth extraction with ridge preservation using mineralized versus combined mineralized-demineralized freeze-dried bone allograft: a randomized controlled clinical trial. J Periodontol. 2015;86(3):348-355.