You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
With some studies reporting survival rates of 92.8% to 97.1% over a 10-year follow-up period, dental implants have become a dependable therapeutic option for both fully and partially edentulous patients.1,2 Despite this success, biologic complications persist and present the general practitioner with a variety of challenges. Thus, practitioners need to be able to recognize the early signs of peri-implant disease and provide patients with reliable treatment options.
From a practical standpoint, it is important to first differentiate peri-implant health from peri-implant disease. Peri-implant health is clinically characterized by the absence of inflammation, radiographic bone loss, mobility, and bleeding on probing.3 The clinician should monitor the periodontal health around the implant and ensure there is no increase in probing depths at each subsequent visit.4
In this context, practitioners should first and foremost recognize and appreciate the difference between early implant failure and late implant failure (peri-implantitis). The former is often characterized as a biological problem and represents the failure of the host tissue to osseointegrate prior to abutment connection. Conversely, late implant failure refers to pathologic bone loss around an osseointegrated implant after prosthetic loading.5
Peri-implant disease manifests initially as peri-implant mucositis, a reversible pathologic condition characterized by the presence of bleeding and/or suppuration on probing and the absence of bone loss. If left untreated, peri-implant mucositis may develop into peri-implantitis, a plaque-associated pathological condition occurring in tissues around dental implants that is characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone.3
In 2017, the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions established well-defined criteria for a definitive diagnosis of peri-implantitis: (1) presence of bleeding and/or suppuration on probing, (2) progressive bone loss, and (3) increased probing depths.3 While these objective markers support diagnostic consistency among practitioners, their clinical application is challenged by the fact that approximately 90% of implants exhibiting peri-implant disease elicit no symptoms to patients.6 The asymptomatic nature of this condition often hinders early diagnosis and prevents patients from seeking care when the disease is at a reversible stage.
The primary objective of this article is to provide the differential diagnosis of peri-implantitis, outlining clinical, radiographic, and histopathologic features of clinically similar benign and malignant lesions that may assist general practitioners in correctly identifying and managing the disease entity. The secondary objective of this article, demonstrated in two selected case studies, is to highlight how practitioners can and should differentiate peri-implant disease from both benign and malignant lesions using clinical and radiographic findings at various stages of the disease sequela.
Materials and Methods
The authors identified resources and publications written in English from the following databases: Google Scholar, PubMed, Embase, and Cochrane Library. This review took into account both experimental and analytical observational investigations, such as cross-sectional studies, case control studies, prospective and retrospective cohort studies, and randomized controlled trials. Systematic reviews and descriptive observational studies, including case series, case reports, and descriptive cross-sectional studies, were also taken into consideration. The exclusion criteria included animal research, editorials, commentaries, poster presentations, and critical assessments of systematic reviews.
Results
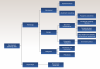
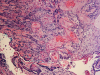
In this review, a total of 10 lesions could be differentiated and outlined as a potential differential diagnosis for peri-implantitis (Figure 1). These lesions can be broadly classified into two categories: physiologic and pathologic entities. Pathologic lesions can be further subcategorized based on their etiology, which includes mechanical, benign, or malignant. Figure 2, for example, depicts squamous cell carcinoma (SCC).
It is crucial for practitioners to consider and include the possibility of these lesions in the diagnostic process of a peri-implant pathology before a definitive diagnosis of peri-implantitis can be established. A summary of clinical, radiographic, and histologic differences is outlined in Table 1.7-27
Case Presentations
Two clinical cases are presented to exemplify the broad spectrum of disease presentations, both benign and malignant, in the differential diagnosis of peri-implantitis. These cases are not meant to serve as a treatment guide but rather as a demonstration of the variability of presentations and the value of a systematic approach for diagnosis.
Case Study 1
Patient Demographics
Patient 1 was a 67-year-old man who presented with the chief complaint of, "I think my implant is loose." The implant (No. 30) had been placed approximately 7 years prior by a provider who was no longer in practice. The patient stated that the onset of his presenting symptoms (ie, implant mobility) was due to an unknown cause that had manifested 8 to 12 months prior. He denied any systemic symptoms of fever or chills and reported no pain or discomfort associated with the implant.
The patient had a medical history significant for human immunodeficiency virus (HIV), which was diagnosed in 1982, a total hip replacement 5 years prior to presentation, and benign prostatic hyperplasia that was diagnosed 2 years prior. He reported no postoperative complications following his hip replacement surgery and stated that his most recent viral load was undetectable. The patient had no known drug allergies and was currently adherent to all medications, which included simvastatin, tamsulosin, oxybutynin chloride, and Genvoya® (elvitegravir, cobicistat, emtricitabine, tenofovir alafenamide).
Clinical and Radiographic Findings
On arrival, the patient was well-appearing and under no acute distress. His extraoral examination was within normal limits, with no asymmetry or lymphadenopathy in the head and neck region. Temporomandibular joint (TMJ) findings were consistent with occasional clicking and popping on the right joint, left-sided deviation (3 mm to 4 mm) on opening, with no joint pain or compromised mobility.
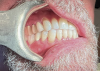
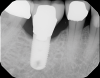
The intraoral orthodontic evaluation showed a class I canine and premolar occlusion on both the right and left sides. The patient had an extensive dental history with multiple fixed prostheses, including both porcelain-fused-to-metal and gold crowns, in the maxillary and mandibular arches. The cement-retained implant crown (No. 30) exhibited 1 mm to 2 mm of mobility in the buccolingual direction. The implant, however, had no signs of acute infection, suppuration, or fistula formation at adjacent and non-adjacent sites (Figure 3).
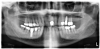
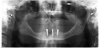
The patient's radiographic assessment, performed immediately following the initial clinical evaluation, included a panoramic radiograph (Figure 4) and bitewing and periapical radiographs of No. 30 (Figure 5). Panoramic findings showed generalized horizontal bone loss confined to the coronal third (15% to 20%), with circumferential bone loss, also referred to as "cupping," around both of the patient's implants, Nos. 30 and 13.
Diagnostic Assessment and Intervention
Among the criteria for implant "success" defined in the literature is marginal bone remodeling of less than 2 mm in the first year and no more than 0.2 mm each year thereafter.28 The patient's physiologic bone loss, negative clinical symptoms, and the fact that the implant was placed more than 7 years ago supported the view that the mobility was likely due to a mechanical failure in the abutment rather than peri-implantitis. Given this background, the patient was referred to a prosthodontist to replace both the broken implant component and cement-retained implant crown. No peri-implant surgical treatment was indicated.
Case Study 2
Patient Demographics
Patient 2 was a well-appearing 74-year-old man who presented with the chief complaint of, "My lower denture is loose." On arrival, the patient stated that he had been edentulous for more than 5 years and was referred by the denturist for implant-supported prosthetic options. His medical history was significant for hypothyroidism and benign prostatic hyperplasia, which were medically controlled. He reported a 40-year history of cigarette smoking but stated that he quit smoking about 6 years prior.
Patient Evaluation and Treatment Progress
On initial presentation, a cone-beam computed tomography (CBCT) scan of the mandible showed sufficient bone to place mandibular implants at a safe distance from the inferior alveolar nerve. Treatment options presented to the patient included two locator-type implants, three locator-type implants, and an "all-on-x" fixed prosthesis. He opted for the utilization of three locator-type implants to improve lower complete denture retention.
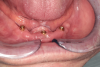
Implants were placed in sites Nos. 27 and 22 (3.8 mm x 9 mm) and site No. 24 (3.8 mm x 10.5 mm). At the 3-month follow-up appointment, all implants were well osseointegrated clinically and radiographically (Figure 6 and Figure 7) with no atypical or pathological findings, and the patient was referred to the denturist for prosthetic rehabilitation.
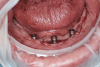
Six months after his last visit, the patient presented with complaints of unresolved pain in the lower gums and a referral from the denturist to assess the mandibular anterior region. The patient reported ongoing pain lasting more than 10 days without resolution despite repeated adjustments by the denturist to relieve areas of irritation. An area of ulceration was noted on the lingual gingiva of implant site No. 24, and a panoramic radiograph showed crestal peri-implant bone loss and thread exposure on all three implants (Figure 8), but this was unrelated to the observed lesion. Percussion of implant No. 24 and palpation of the buccal aspect did not yield a painful response. The tissue cuff surrounding the locator-type abutment was sensitive to palpation and localized to the lingual frenum adjacent to the implant.
The No. 24 locator-type abutment was removed to assess the site thoroughly, but no significant additional findings were noted. The lesion was localized to the lingual aspect of implant site No. 24, was non-homogenous in color, and had a granular surface topography (Figure 9). The initial diagnosis at the time of presentation was determined to be a traumatic ulcer caused by an overextended lingual flange. The lingual flange was completely removed from the lower denture. Although the site did not demonstrate any signs of active infection, antibiotic prophylaxis was prescribed to prevent invasion of opportunistic organisms into areas of the wound. Prescriptions for Kenalog/Orabase, dexamethasone, and amoxicillin were provided to the patient to address the inflammatory reaction. He was instructed to refrain from using the lower denture until the lesion was properly identified and treated.
At the 2-week follow-up examination, no improvements were observed in the clinical or radiographic appearance of the lesion. As a result, it was deemed necessary to refer the patient to an oral surgeon for a comprehensive evaluation and biopsy of the lesion.
A biopsy taken from the floor of the mouth and a histopathological examination yielded positive results consistent for SCC. The patient was referred to a cancer center for further treatment. He was staged with SCC with no metastasis. The treatment plan included a local resection without radiation, bilateral neck dissection, and removal of implants.
Discussion
Peri-implantitis is a pathological condition characterized by inflammation and destruction of the soft and hard tissues surrounding dental implants.5 The differential diagnosis of peri-implantitis includes a wide range of conditions that may present with similar clinical and radiographic features. To properly categorize these lesions, it is necessary to apply a systematic approach that considers the distinguishing features of each entity.
The first diagnostic step is to distinguish between physiologic and pathologic lesions (Figure 1). An implant is considered to be "successful" if marginal bone remodeling is less than 2 mm in the first year and no more than 0.2 mm each year thereafter.5,28 Some bone loss is to be expected and does not necessarily indicate an ongoing pathologic resorptive process. Clinicians should always assess the level of peri-implant bone loss using sequential radiographs, ensuring baseline references are available to monitor progress at subsequent postoperative follow-ups.29 If the rate of bone loss exceeds the range of acceptability, further assessment and referral to a specialist is recommended.
Pathologic entities on the differential can be broadly categorized as mechanical, benign, or malignant. Mechanical failures are often due to either a loosened abutment in the implant fixture or fracture of the abutment screw or the fixture itself. Loose abutments rarely present with clinical symptoms of pain and/or inflammation around the implant site. The management strategy is to identify the etiology prior to replacing the restorative component. Implant fractures, conversely, can cause pain and may require referral to an oral surgeon for implant removal. When assessing for implant fractures, it is useful to look for mitigating risk factors as potential sources of the problem. These risk factors include pocket depths greater than 5 mm, parafunctional habits (eg, bruxism), implant diameter less than 4 mm, unfavorable crown-to-implant ratio, cantilevers, and an excessive occlusal table size resulting in enhancement of destructive extra-axial forces.11
In this regard, the patient in the first case study presented with the sole symptom of "implant mobility." The absence of other clinical symptoms-only a physiological level of bone resorption over the course of 7 years-and the positive finding of buccolingual mobility pointed to a loosened abutment as the cause of the chief complaint. In this case, the patient was appropriately referred to a prosthodontist for evaluation and eventual abutment replacement.
Benign lesions on the differential can be subcategorized as either reactive/inflammatory or potentially malignant. Reactive lesions are typically well-circumscribed and painless, with little hard-tissue involvement. Occasionally, overlapping characteristics such as friability and ulceration make clinical distinctions challenging, in which case it is recommended that the lesion be biopsied to rule out malignancy.
The preferred treatment modality for benign reactive/inflammatory lesions, such as pyogenic granuloma, peripheral ossifying fibroma, and peripheral giant cell granuloma, is excision with or without curettage and decontamination of the exposed implant surface. According to an investigation by Román-Quesada et al, all of these lesions show a relatively high recurrence rate (30% to 40%) compared to similar lesions around teeth (15% to 20%).30 This rate of recurrence is not affected by curettage and does not vary among the different benign lesions. After excision, providers should reinforce oral hygiene instructions with patients.15 If recurrence is high or bone loss exceeds physiologic norms, referral to an oral surgeon may be warranted for evaluation and removal of the involved implant.
Certain benign lesions, such as oral lichen planus (OLP), are classified as potentially malignant and share many similarities to malignant lesions. For example, erosive OLP appears as an erythematous and ulcerated lesion with a background of reticular white striae. Gonzáles-Moles et al reported that the rate of malignant transformation of OLP in different studies was established within a margin between 0% and 12.5% with implant placement notably exacerbating the disease process.20,31 OLP is considered a chronic inflammatory autoimmune disorder and, if the effect of dental implants on the immune response and the development of chronic inflammation is considered, it is reasonable to suggest that they could trigger the malignant transformation of these types of lesions.31 This malignant transformation is more likely to occur in atrophic and/or erosive lichen planus lesions.31 As a result, such lesions require long-term follow-up and appropriate referrals to a specialist for management.32 Therefore, practitioners should conduct routine head and neck examinations on all patients to identify dysplastic lesions early in the disease course. Counseling patients with modifiable risk factors, such as tobacco and alcohol consumption, may improve long-term outcomes.33
Malignant lesions can be primary (eg, squamous cell carcinoma) or metastatic, with the most common sites for primary SCC being the lateral border of the tongue and the floor of the mouth.34 The effect of dental implants on malignant transformation of surrounding mucosa is heavily debated. One leading theory considers the constant inflammation experienced by the tissue resulting in tissue instability.35 This process may be compounded by exposure to other known risk factors, such as cigarette smoking or alcohol consumption. Due to the similarities in clinical/radiographic appearance to peri-implantitis, these lesions are often misdiagnosed, leading to a critical delay in treatment.36 The most frequent primary tumors that metastasize to the oral cavity in women are those located in the breast (42%), while in men, the most frequent are those in the lung (22.3%) and prostate (12%).37 Gnathic bone involvement most commonly originates from breast malignancies, whereas oral soft-tissue metastases usually originate from the lung.25
Orhan et al described a case of metastasis in a 69-year-old patient treated for breast cancer 18 years earlier undergoing annual check-ups, with no signs of disease recurrence. Two dental implants were placed in the mandibular premolar area, and 3 months later the patient developed numb chin syndrome, along with chest pain, arthralgia, back pain, vomiting, and perspiration that, once studied, confirmed a recurrence of breast cancer, with metastasis in different locations, including around the implants.38 The attached gingivae are the most frequently affected sites (54%) in oral soft-tissue metastasis.25 Clinically, these lesions are often painful, erythematous, and/or ulcerated.25 Radiographically, malignant lesions are more likely to exhibit bone destruction with ill-defined borders. In certain cases, radiographs may show a "moth eaten" appearance or spiculated periosteal reactions consistent with neoplastic involvement of the cortical bone.25 Providers must correlate aforementioned clinical and radiographic findings with patient history to identify the need for biopsy in such cases. Early identification and referral to an oral surgeon for biopsy/further management is crucial when malignancy is suspected.
With these crucial factors in mind, a review of the second case study shows that the patient presented 6 months after implant placement with persistent pain on palpation lingual to site No. 24 despite numerous adjustments and complete removal of the lingual flange by the denturist. Initially, the authors' approach focused on addressing the perceived cause, treating the lesion as a traumatic ulcer with corticosteroids and denture adjustment. However, considering the patient's social history and nicotine abuse, the possibility of oral SCC was contemplated.
Local factors, such as trauma or surgical procedures, could further increase the differential diagnosis process because of the trap of tumor cells during the formation of clots in recent wounds and growth factors locally released by regenerated tissues, which would stimulate the formation and proliferation of malignant tumor cells.39 Chronic inflammation also promotes metastasis locally because circulating tumor cells can become trapped within the rich capillary network of tissues that exhibit chronic inflammation.39
Thus, it is strongly advised that general dentists record a radiograph of the implant site to support their clinical findings40 and be cognizant of other factors that may impact implant success, including patient factors such as smoking, medical conditions, and medications. In cases where patients show no improvement during follow-up, suspected lesions should always be referred to specialists for biopsy and appropriate management.
Conclusion and Recommendations
The differential diagnosis of peri-implantitis includes a wide range of conditions, both benign and malignant, that often exhibit similar clinical and radiographic features. The presented case studies underscore the significance of a systematic approach in diagnosing and differentiating peri-implantitis from other lesions on the differential. Of particular importance is the identification and management of malignant and potentially malignant entities that require thorough understanding of the patient's individualized risk factors. By promptly recognizing and accurately diagnosing peri-implant lesions, practitioners can initiate timely intervention and develop appropriate treatment plans, leading to optimal patient outcomes. Upon the appearance of lesions compatible with peri-implantitis, a differential diagnosis should be made with SCC, and in cases of the sudden appearance of hyperplastic or osteolytic lesions that are unresponsive to conventional treatment and/or anesthesia or paresthesia, a biopsy is crucial. Peri-implant metastases and primary tumors are, in general, rare, but highly atypical or refractory reaction to the treatment of peri-implantitis is suspicious for malignancy as long as the contrary remains unproven.
Acknowledgment
The authors recognize and thank Arthi Kumar, DDS, for her contributions in providing histopathologic images of the malignant lesions included in this article, and Albert Lui, DDS, and Andres Marquez-Guzman, DDS, for their contributions in the second case study.
About the Authors
Niam Kataria, DDS Candidate
New York University College of Dentistry, New York, New York
Alireza Hatamifar, MS, DDS
First Year Postgraduate, Department of Oral and Maxillofacial Surgery, University of California San Francisco Medical Center, San Francisco, California
Jazmin Lui, DDS
Private Practice, Calgary, Alberta, Canada
Denise Trochessett, DDS
Clinical Professor and Chair, Department of Oral and Maxillofacial Pathology, Radiology, and Medicine, New York University College of Dentistry, New York, New York
Thomas G. Wiedemann, MD, PhD, DDS
Clinical Associate Professor, Department of Oral and Maxillofacial Surgery,
New York University College of Dentistry, New York, New York
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Hämmerle CH, Stone P, Jung RE, et al. Consensus statements and recommended clinical procedures regarding computer-assisted implant dentistry. Int J Oral Maxillofac Implants. 2009;24 suppl:126-131.
2. Ting M, Craig J, Balkin BE, Suzuki JB. Peri-implantitis: a comprehensive overview of systematic reviews. J Oral Implantol. 2018;44(3):225-247.
3. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 suppl 20:S286-S291.
4. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018;45 suppl 20:S246-S266.
5. Cochran D. Implant therapy I. Ann Periodontol. 1996;1(1):707-791.
6. Rokaya D, Srimaneepong V, Wisitrasameewon W, et al. Peri-implantitis update: risk indicators, diagnosis, and treatment. Eur J Dent. 2020;14(4):672-682.
7. Geraets W, Zhang L, Liu Y, Wismeijer D. Annual bone loss and success rates of dental implants based on radiographic measurements. Dentomaxillofac Radiol. 2014;43(7):20140007.
8. Degidi M, Perrotti V, Piatelli A, et al. Histology of a dental implant with a platform switched implant-abutment connection. J Osseointegration. 2011;3(3):56-60.
9. Minkin C, Marinho VC. Role of the osteoclast at the bone-implant interface. Adv Dent Res. 1999;13:49-56.
10. Marcelo CG, Filié Haddad M, Gennari Filho H, et al. Dental implant fractures - aetiology, treatment and case report. J Clin Diagn Res. 2014;8(3):300-304.
11. Sánchez-Pérez A, Moya-Villaescusa MJ, Jornet-García A, Gomez S. Etiology, risk factors and management of implant fractures. Med Oral Patol Oral Cir Bucal. 2010;15(3):e504-e508.
12. Shnaiderman-Shapiro A, Dayan D, Buchner A, et al. Histopathological spectrum of bone lesions associated with dental implant failure: osteomyelitis and beyond. Head Neck Pathol. 2015;9(1):140-146.
13. Sánchez-Torres A, Mota I, Alberdi-Navarro J, et al. Inflammatory fibro-epithelial hyperplasia related to a fixed implant-supported prosthesis: a case report. J Clin Exp Dent. 2018;10(9):e945-e948.
14. Dojcinovic I, Richter M, Lombardi T. Occurrence of a pyogenic granuloma in relation to a dental implant. J Oral Maxillofac Surg. 2010;68(8):1874-1876.
15. Atarbashi-Moghadam F, Atarbashi-Moghadam S, Namdari M, Shahrabi-Farahani S. Reactive oral lesions associated with dental implants. A systematic review. J Investig Clin Dent. 2018;9(4):e12342.
16. NagamineH, Yasui T, Kimura M, et al. Large pyogenic granuloma associated with a dental implant: a case report. J Oral Maxillofac Surg Med Pathol. 2022;34(3):315-321.
17. Brown AL, Camargo de Moraes P, Sperandio M, et al. Peripheral giant cell granuloma associated with a dental implant: a case report and review of the literature. Case Rep Dent. 2015:2015:697673.
18. Mohiuddin K, Priya NS, Ravindra S, Murthy S. Peripheral ossifying fibroma. J Indian Soc Periodontol. 2013;17(4):507-509.
19. Mishra MB, Bhishen KA, Mishra S. Peripheral ossifying fibroma. J Oral Maxillofac Pathol. 2011;15(1):65-68.
20. Agha-Hosseini F, Rohani B. Evaluation of the effects of dental implants on oral lesions. J Contemp Dent Pract. 2015;16(5):400-406.
21. Górski B. Dental implant treatment in patients suffering from oral lichen planus: a narrative review. Int J Environ Res Public Health. 2022;19(14):8397.
22. Kerr AR, Robinson ME, Meyerowitz C, et al; National Dental PBRN Collaborative Group. Cues used by dentists in the early detection of oral cancer and oral potentially malignant lesions: findings from the National Dental Practice-Based Research Network. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):264-272.
23. Bhatavadekar NB. Squamous cell carcinoma in association with dental implants: an assessment of previously hypothesized carcinogenic mechanisms and a case report. J Oral Implantol. 2012;38(6):792-798.
24. Müller S, Boy SC, Day TA, et al. Data set for the reporting of oral cavity carcinomas: explanations and recommendations of the Guidelines from the International Collaboration of Cancer Reporting. Arch Pathol Lab Med. 2019;143(4):439-446.
25. Kumar G, Manjunatha B. Metastatic tumors to the jaws and oral cavity. J Oral Maxillofac Pathol. 2013;17(1):71-75.
26. Dib LL, Soares AL, Sandoval RL, Nannmark U. Breast metastasis around dental implants: a case report. Clin Implant Dent Relat Res. 2007;9(2):112-115.
27. Gaver A, Polliack G, Pilo R, et al. Orofacial pain and numb chin syndrome as the presenting symptoms of a metastatic prostate cancer. J Postgrad Med. 2002;48(4):283-284.
28. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
29. Bergamini M, Gomez J, Talib HS, et al. An algorithmic approach to evaluation of an ailing implant. Compend Contin Educ Dent. 2020;41(8):e1-e9.
30. Román-Quesada N, González-Navarro B, Izquierdo-Gómez K, et al. An analysis of the prevalence of peripheral giant cell granuloma and pyogenic granuloma in relation to a dental implant. BMC Oral Health. 2021;21(1):204.
31. Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14(3):229-243.
32. Tsushima F, Sakurai J, Uesugi A, et al. Malignant transformation of oral lichen planus: a retrospective study of 565 Japanese patients. BMC Oral Health. 2021;21(1):298.
33. Garcia de Sousa FA, Blumer Rosa LE. Oral lichen planus: clinical and histopathological considerations. Braz J Otorhinolaryngol. 2008;74(2):284-292.
34. Jané-Salas E, López-López J, Roselló-Llabrés X, et al. Relationship between oral cancer and implants: clinical cases and systematic literature review. Med Oral Patol Oral Cir Bucal. 2012;17(1):e23-e28.
35. Ramos JC, Dos Santos ES, Costa Normando AG, et al. Oral squamous cell carcinoma around dental implants: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(6):660-674.
36. Markopolous AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126-130.
37. Moergel M, Karbach J, Kunkel M, Wagner W. Oral squamous cell carcinoma in the vicinity of dental implants. Clin Oral Investig. 2014;18(1):277-284.
38. Orhan K, Bayndr H, Aksoy S, et al. Numb chin syndrome as a manifestation of possible breast cancer metastasis around dental implants. J Craniofac Surg. 2011;22(3):942-945.
39. Salgado-Peralvo AO, Serrano-Sánchez V, Vaello-Checa I, et al. Cancerous lesions in the vicinity of dental implants: a systematic review. J Oral Med Oral Surg. 2020;26(4):45.
40. Wiedemann T, Hatamifar A, Jung A, Talib H. Peri-implantitis: a comprehensive overview for the general dental practitioner. J Dent Oral Sci. 2022;4(4):1-10.