You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Various "graftless" approaches to implant placement can be used when treating the severely atrophied maxilla. As discussed in Part 1 (Compendium, February 2023), such approaches incorporate alternative implant designs that maximally utilize these patients' residual bone structure and volume. Such unconventional implants can serve as solutions without the use of, or using only minimal, bone grafting, thus reducing treatment times, costs, and potential complications. This article evaluates current state-of-the-art graftless implantology strategies as alternatives to extensive bone grafting procedures for the rehabilitation of the severely atrophic maxillary arch. While Part 1 discussed short and narrow-diameter implants, nonalveolar anatomical and tilted implants, and pterygoid implants, this second article specifically covers zygomatic implants and individualized subperiosteal implants and provides general recommendations. (Part 1 also described the literature search methodology for this review.)
Zygomatic Implants
Review Results
Zygomatic implants are inserted through the alveolar crest and maxillary sinus to engage the malar bone.1 They are 30 mm to 52.5 mm long with diameters of 4 mm at the apical portion and 4.5 mm to 5 mm at the alveolar one-third.1,2 These implants have roughened and oxidized surfaces with a smooth mid-body, self-tapping apical ends, and a 55-degree angulation at the head to maintain perpendicularity to the occlusal surface.1,2
Zygomatic implants are mainly indicated in completely edentulous patients with severely resorbed ridges and pneumatized sinuses in the posterior region. Specifically, edentulous patients with bone in only the anterior maxillary region and lack of bone on each posterior region are candidates for full-arch restorations with conventional implants in the anterior maxillary region and bilateral zygomatic implants (Figure 1).2 Edentulous patients with lack of bone in both the anterior maxillary and posterior regions are candidates for four zygomatic implants, also known as quad zygoma treatment.2 The use of zygomatic implants is also a treatment option for patients with defects from trauma and resective surgeries due to pathology.3
Patients treated with zygomatic implants may benefit from immediate rehabilitation following a single surgery. Zygomatic implants can attain maximum stability and success by reducing cantilever stress with cross-arch stabilization and engaging in four cortices (the palatal cortex of the maxillary alveolus, the cortical floor of the maxillary sinus, and the two zygomatic bone cortices at the apex of the implant).1 Zygomatic implants can provide immediate functional rehabilitation in diverse clinical situations such as the rehabilitation of the severely resorbed, traumatized, and therapeutically resected maxilla. This unique approach to dental and orofacial rehabilitation using dental implants in an area that would otherwise be inaccessible is direct and efficient when compared with grafting solutions.
Reported possible limitations associated with zygomatic implants include complex surgical placement that requires advanced training and the involvement of general anesthesia or deep sedation.1 The restorative phase of treatment can also be complicated, demanding the resolution of palatally located abutments.4 Zygomatic implants differ from conventional ones in biomechanics, clinical procedures, outcomes, and eventual complications. A significant incidence of complications such as maxillary sinusitis, possible soft-tissue complications around abutments, oro-antral fistula formation, damage to the orbital contents, and eventual paresthesia/dysesthesia has been reported.3 The most concerning potential problem is that the possible failure of a zygomatic implant requires more complicated treatments in contrast to the failure of conventional implants.
Discussion
The use of zygomatic implants should be pre-empted with an adequate otolaryngology specialist consultation for the evaluation of the health of the sinuses before treatment is initiated. Patients with a previous history of recurrent rhinosinusitis are not candidates for zygomatic implant therapy and such a condition will be further exacerbated by the placement of trans-sinus zygomatic implants. In cases of sinus infections after the placement of zygomatic implants, functional endoscopic sinus surgery is indicated to provide drainage of sinus infections that are refractory to antibiotic treatment.5
Zygomatic implants are being increasingly used as a "rescue" option for conventional implants that have failed to integrate or failed over time.5 In particular, in cases where a conventional implant that is part of a full-arch restoration fails, a zygomatic implant can be immediately placed as a replacement for the failed implant to continuously maintain the full-arch fixed prosthesis. This is especially favorable since such edentulous patients are offered the benefit of being able to maintain their prosthesis even under circumstances of failures of their conventional implants.
The success rates of zygomatic implants with follow-up periods ranging from 12 to 48 months have been reported as high as 98.1%.2 Esposito and Worthington did not identify any randomized controlled trials investigating whether zygomatic implants offer some advantages over alternative techniques like bone augmentation or sinus augmentation with bone or biomaterials.6 In 2018, Aboul-Hosn Centenero et al published an article where survival rates of two zygomatic implants combined with standard implants in the anterior maxillae were compared to quad zygoma therapy outcomes.7 This was the first article directly comparing the two protocols and showed no difference between the protocols with regard to implant survival. The data analysis clearly showed comparable results for the quad zygoma protocol compared to the two zygomatic plus standard implants. Such high survival rates further favor zygomatic implants over traditional bone grafting procedures such as sinus lifts and major autogenous bone grafting procedures and their comparatively significant failure rates. Scientific evidence demonstrates that the technique is reliable and predictable. For the clinician, though, excellent clinical expertise with a thorough knowledge of the anatomy in three dimensions is necessary, along with an understanding of the physiology of the sinus and the biomechanics of the prosthetic reconstruction.
Individualized Subperiosteal Implants
Review Results
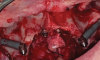
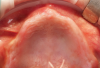
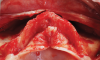
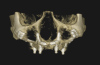
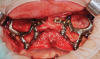
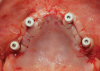
Subperiosteal implants comprise metal frameworks individually fabricated to adapt to and restore edentulous areas. Custom-made and designed to fit the patient's unique bony morphology, subperiosteal implants are placed below the periosteum and stabilized by fixation screws that contact the underlying bone and fibrous tissue that covers them.8 Unlike conventional implants, subperiosteal implants rest directly on top of bone, with rods protruding through the gingiva to accommodate fixed or removable complete or partial prostheses. (A case presentation of individualized subperiosteal implants is depicted in Figure 2 through Figure 10).
The patient's bony anatomy is first scanned using CBCT technology, and the data is utilized for a virtual reconstruction of the patient's edentulous jaw. This data is then combined with information from intraoral scans and diagnostic wax-ups to produce a virtual 3D model and properly plan the designs of the implant and prosthesis. Modern subperiosteal implants are usually manufactured through direct metal laser sintering (DMLS), where a high-power laser is used to melt metallic powders layer by layer. A replica of the implant is also manufactured in resin material so the clinician can properly plan the surgery in regard to the locations of the vital anatomical structures and appropriate flap design.
During the day of delivery, after sufficient exposure of the underlying anatomical buttresses is achieved, the implant is checked for proper adaptation, and any necessary modifications are made before final insertion. The implant is stabilized in place with monocortical screws inserted through predetermined holes in the underlying bone. The flap is properly dissected to gain sufficient passivity to achieve primary intention closure around the exposed implant abutments.8 After the necessary healing period, the proper impressions are taken for final prosthesis manufacturing and delivery.
Recent advances in CBCT technology and imaging precision have reduced treatment time to a single surgical session, as skeletonization of the patient's residual jaw is no longer necessary for the fabrication of the prosthesis. 3D printing and, in particular, DMLS also allow for fabrication of custom-made meshes and implants that can perfectly adapt to the patient's specific anatomy. These qualities have stimulated interest in the use of subperiosteal implants especially for the management of patients with complex atrophies and histories of trauma and/or tumor ablation/resection, where graftless implant options may not be plausible.9 In any case, surgery for placement of a subperiosteal implant is technically more complex than the classic positioning of endosseous implants. Therefore, the clinician's surgical skills are a factor in implant insertion and management of any possible biological and technical complications.
Still, this treatment option can be beneficial for patients with limited finances who do not wish to undergo complex regenerative therapies or are not candidates for extended treatment times for oral rehabilitation.8 Well-adapting custom-made subperiosteal implants can now be delivered as solutions for virtually any individual extreme situation.8-12
Discussion
Starting around the middle of the 20th century, subperiosteal implants were used as custom-made fixtures inserted below the periosteum. They were stabilized by contact with the underlying bone by means of fixation screws and the fibromucous tissue that covered them. Usually made of cobalt-chrome or titanium alloys, they were restored by means of transmucosal abutments that emerged above the periosteum, inside the oral cavity. Because of the need to capture a physical impression of the residual bone, the technical fabrication of subperiosteal implants was complex. The skeletonization during the preliminary surgical session caused significant patient discomfort, and the impressions were often inaccurate.8
The subsequently manufactured subperiosteal implants that were based on these impressions were far from precise, which raised the risk of achieving unpredictable clinical results. In fact, the need to adapt these implants during the second surgical session often led to lengthy procedures, which increased the risk of infections and complications. This led to insufficiently fixed subperiosteal implants that caused progressive bone loss and further worsening of the atrophic situation.10 Unpredictable clinical results, such as soft-tissue complications, implant exposure, implant failure, and even implant loss, were common. While subperiosteal implants were used for several years, the difficulty in properly positioning them along with high complication rates led to their replacement by endosseous, root-form dental implants.
Digital technology has now spurred a new era in dentistry. From advanced acquisition methods such as CBCT, to intraoral scanners, digital software, and 3D printers, modern tools have simplified, improved, and sped up various procedures. 3D printing and DMLS enables fabrication of custom-made meshes and implants that can adapt perfectly to the patient's specific anatomy, allowing for older concepts such as the placement of subperiosteal implants to be revisited and revitalized under new technological contexts consolidated with anatomical and physiological principles. The ability to provide single surgical session treatment and achieve enhanced precision reduces costs for the patient and increases predictability and safety in the short term. Thus, subperiosteal implants are now an attractive option for the management of patients with complex atrophies and histories of trauma and tumor ablation/resection where graftless implant treatments may not be plausible.9
CBCT allows acquisition of highly accurate 3D data of the patient's residual bone volume in the area of interest. This data is re-elaborated with the appropriate reconstruction software, enabling generation of a virtual model of the patient's atrophic jaw. Capturing an optical impression of the dentate arches allows for modeling of a virtual diagnostic wax-up. Finally, the superimposition of the jaw and dental models, together with the virtual wax-up, facilitates the design and modeling of custom-made subperiosteal implants. These are designed and conceived specifically for the patient's needs, both as a bone-supported structure and a prosthetic emergency. These 3D-printed implants represent a possible alternate solution for the rehabilitation of atrophic arches and for patients who do not want to or cannot undergo traditional regenerative techniques preparatory to the insertion of conventional endosseous implants.
Increasingly advanced DMLS techniques, in particular, allow the older concept of subperiosteal implants to be revisited. DMLS, which arose in the late 1990s and has since gained importance in dentistry, is an additive manufacturing technique that uses a high-power laser to melt metallic powders together. The procedure involves the construction of a stratified 3D model of the implant, layer by layer. The CAD file is sliced into thin layers, creating a series of 2-dimensional images that, when laced together, compose the 3-dimensionality of the product.13 The laser beam then selectively melts the powders of each layer, and the process is continuously repeated layer by layer, until completion of the manufacturing of the device. With DMLS, there is almost no limitation in the fabrication of complex objects. Both standard endosseous and custom-made subperiosteal titanium or titanium alloy implants may be manufactured. Surovas demonstrated the feasibility and economic sustainability of designing and manufacturing custom-made subperiosteal implants in titanium alloy via DMLS that were able to adapt perfectly to the 3D-printed bone model.14 The steps involved in the design and fabrication of these custom-made implants are: computerized tomography (CT) scan of the area of interest, processing of the CT data, creation of a 3D virtual model of the bony anatomy, modeling technique for the custom implant, and preparation of the data file for 3D printing. The custom-made subperiosteal implant is then fabricated in Ti6Al4V material using DMLS.14
Cohen et al developed custom-made subperiosteal Ti6Al4V devices with additive manufacturing and added osteogenic micro- and nanoscale surface texture modification features.15 The porous surfaces of these implants demonstrated the potential to stimulate human osteoblasts to produce osteogenic factors, and eventually high bone-to-implant contact was found for DMLS discs implanted in rat calvaria and rabbit tibia. When implanted in the human posterior mandible, 3- and 8-month postoperative images showed new bone formation and osseointegration. Such discoveries of the osteogenic potential of subperiosteal implants manufactured through DMLS technology have contributed to their increasing use.
Experience with and knowledge of CAD software is crucial when producing the full digital design of these implants. Shortcomings still exist such as potential scattering distortions from nearby crowns or teeth in the CBCT scans.8,11 Therefore, criticalities can emerge in the implant design in the presence of a CBCT scan with artifacts or substantial scattering in the area to be reconstructed, which can cause complexities and requires adequate CAD knowledge. Some company services now offer assistance for surgeons at relatively low costs; these companies typically own the software and machines used to manufacture the implants and, in this sense, represent the best solution for the clinician. In any case, surgery for placement of a subperiosteal implant is technically more complex than the classic positioning of endosseous implants. The clinician's surgical skills play a fundamental role in the insertion of the implant(s) and management of any potential biological and technical complications. The re-emergence of subperiosteal implants in light of a new technological context is still relatively fresh and the advantages and disadvantages of this process are not yet clearly defined. This treatment option, however, may be a favorable alternative to complex regenerative therapies.8
General Recommendations and Overall Approach
Rehabilitation of maxillary atrophy with dental implants is clinically challenging despite the wide variety of surgical techniques available. Finding the right indication for a procedure is critical for the long-term stability of the implant(s). With the introduction of the concept of "teeth-in-a-day," clinicians have explored innovative techniques to attain the goal of immediate implant-supported provisional prostheses. However, costs and comorbidities are limitations to the advancement of these techniques.
Because of the invasive nature of dental implant therapy and the virtual impossibility of randomizing treatment groups, the success rates of graftless implant solutions compared to conventional surgical implant therapy is based mainly on prospective and retrospective cohort studies instead of more scrutinizing randomized controlled trials. Thus, there is a need to obtain studies with longer follow-up times. However, there are many oral amputees who desire and require restorations and should be treated utilizing the recent advancements in graftless implant dentistry.
Today, clinicians are able to provide immediately loaded prostheses even for patients with the most unfavorable bony conditions who desire prosthetic rehabilitation. Still, a comprehensive, multifactorial assessment of every individual patient's situation is necessary, and no assessment should ever be compromised, no matter how desperate the situation appears. Like any surgery, graftless implant procedures come with risks due to their invasive nature.
Initially, the remaining bony anatomy should be thoroughly assessed and measured to determine whether the edentulous maxilla can be completely restored with conventional implants, including minimal to moderate bone grafting procedures, or with multiple short and/or narrow-diameter implants. If these options are not possible but there is still adequate extra-alveolar bone in the paranasal region, interantral implants can offer excellent stability with less invasiveness.
In instances with severe maxillary sinus pneumatization but adequate bone in the paranasal or maxillary anterior region, two paranasal implants or two maxillary anterior conventional implants can be combined with two bilateral pterygoid or zygomatic implants for a full-arch restoration. In instances with extreme maxillary sinus confluence with the nasal fossa leading to a total lack of paranasal bone, four zygomatic implants, also known as quad zygoma treatment, can be utilized for a complete arch restoration.
For patients with a significant medical or surgical history due to major tumor and pathology ablations that contraindicate the previously mentioned graftless implants, individually fabricated subperiosteal implants may still stand as an eventual solution.
Conclusion
Advances in implant design and therapeutics over the past several decades have challenged the traditional concepts of implant anchorage in extremely resorbed residual alveolar ridges, the notion of axial biomechanics, and the process of osseointegration. The use of "graftless" pterygoid, zygomatic, tilted, short, small-diameter, and individualized implants has provided novel ways of achieving implant osseointegration to support dental prostheses in cases of extreme atrophy. The modern development of diagnostic imaging and 3D printing technologies has aided these treatment modalities, and studies now cite a high degree of success for their usage. Nonetheless, these types of implants all have limitations and unique complications that must be considered during planning and at the time of placement to limit potential risks.
Because these nongrafting solutions, however, circumvent local and second-site morbidity, reduce treatment time, and may be aligned with immediate loading protocols, they offer selected benefits for implant rehabilitations. When conventional implant dentistry reaches its limits, customized digitally engineered solutions may allow for an innovative line extension and offer a strategy for implant-borne dental rehabilitation in the future. More understanding is required to determine the optimal combination of different graftless implant solutions for predictable and feasible restorations in highly atrophic maxillary arches. Until this is achieved, implant surgeons should consider the advantages and disadvantages of each technique for every clinical situation and select the approach with the most manageable costs, lowest morbidity, and greatest chance of success.
Acknowledgment
The authors thank and are very grateful to Mr. Rod Jacinto, Sales Director Europe, BoneEasy, for his contributions of the images of individualized patient-specific implants depicted in Figure 2 through Figure 10.
About the Authors
Sung Hoon Choo, DDS
General Practice Residency, Kings County Hospital Center, Brooklyn, New York
Robert S. Glickman, DMD
Associate Dean for Clinical Affairs and Hospital Relations, Professor and Chair, Department of Oral and Maxillofacial Surgery, New York University College of Dentistry, New York, New York
Thomas G. Wiedemann, MD, PhD, DDS
Clinical Associate Professor, New York University College of Dentistry, New York, New York
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Sharma A, Rahul GR. Zygomatic implants/fixture: a systematic review. J Oral Implantol. 2013;39(2):215-224.
2. Aparicio C, Manresa C, Francisco K, et al. Zygomatic implants: indications, techniques and outcomes, and the zygomatic success code. Periodontol 2000. 2014;66(1):41-58.
3. Davó R, David L. Quad zygoma: technique and realities. Oral Maxillofac Surg Clin North Am. 2019;31(2):285-297.
4. Chaware SH, Thakare V, Chaudhary R, et al. The rehabilitation of posterior atrophic maxilla by using the graftless option of short implant versus conventional long implant with sinus graft: a systematic review and meta-analysis of randomized controlled clinical trial. J Indian Prosthodont Soc. 2021;21(1):28-44.
5. Bedrossian E. Rescue implant concept: the expanded use of the zygoma implant in the graftless solutions. Oral Maxillofac Surg Clin North Am. 2011;23(2):257-276, vi.
6. Esposito M, Worthington HV. Interventions for replacing missing teeth: dental implants in zygomatic bone for the rehabilitation of the severely deficient edentulous maxilla. Cochrane Database Syst Rev. 2013;2013(9):CD004151.
7. Aboul-Hosn Centenero S, Lazaro A, Giralt-Hernando M, Hernandez-Alfaro F. Zygoma quad compared with 2 zygomatic implants: a systematic review and meta-analysis. Implant Dent. 2018;27(2):246-253.
8. Mangano C, Bianchi A, Mangano FG, et al. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: a case series. 3D Print Med. 2020;6(1):1. doi: 10.1186/s41205-019-0055-x.
9. Mian M, Delpachitra S, Ackland D, et al. Three‐dimensional printing in oral and maxillofacial surgery: current landscape and future directions. Oral Surgery. 2022;15(3):431-442.
10. Gellrich NC, Rahlf B, Zimmerer R, et al. A new concept for implant-borne dental rehabilitation; how to overcome the biological weak-spot of conventional dental implants? Head Face Med. 2017;13(1):17.
11. Cerea M, Dolcini GA. Custom-made direct metal laser sintering titanium subperiosteal implants: a retrospective clinical study on 70 patients. Biomed Res Int. 2018;2018:5420391. doi: 10.1155/2018/5420391.
12. Gellrich NC, Zimmerer RM, Spalthoff S, et al. A customised digitally engineered solution for fixed dental rehabilitation in severe bone deficiency: a new innovative line extension in implant dentistry. J Craniomaxillofac Surg. 2017;45(10):1632-1638.
13. Mangano F, Chambrone L, van Noort R, et al. Direct metal laser sintering titanium dental implants: a review of the current literature. Int J Biomater. 2014;2014:461534. doi: 10.1155/2014/461534.
14. Surovas A. A digital workflow for modeling of custom dental implants. 3D Print Med. 2019;5(1):9.
15. Cohen DJ, Cheng A, Kahn A, et al. Novel osteogenic Ti-6Al-4V device for restoration of dental function in patients with large bone deficiencies: design, development and implementation. Sci Rep. 2016;6:20493. doi: 10.1038/srep20493.