You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
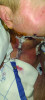
Jeff Chamberlain, DC, seemingly had it all. At 55, he was married to his college sweetheart and a proud father to two active, intelligent, compassionate, driven young adults (Figure 1). He was a successful chiropractic physician, working side-by-side with his wife, Jill, a practice wellness facilitator and teacher. In June 2021, however, their lives changed when Jeff noticed gingival pain and sought the opinion of a dentist. At first, it was believed to be a tooth infection, so mitigating steps were taken and a follow-up visit was scheduled for 6 weeks later. During that time, however, the symptoms were not resolved; in fact, the area around the gingiva began to bleed, and Jeff also detected an enlarged gland on the side of his neck. At the follow-up appointment, the dentist shared his concern and suggested an immediate consultation/evaluation by an oral surgeon, which resulted in a biopsy. The following week, Jill received an urgent call from the oral surgeon's office, informing her that the results of the biopsy were ready and that they needed to see him immediately. The preliminary diagnosis was oral cancer. A consultation was scheduled with a head and neck surgeon, and Jeff then began 2 weeks of grueling biopsies, tests, and scans, the results of which confirmed that he had Stage 4 Squamous Cell Carcinoma of the head and neck. The cancer was very aggressive, so almost immediately, Jeff endured 12-plus hours of surgery, resulting in a partial maxillectomy, a neck dissection, and the removal of some of his lower mandible (Figure 2).
Oral cancer is one of the most fascinating challenges that the dental profession faces. How do we identify this devastating disease before it becomes advanced? This article will detail three key points for diagnosing oral cancer and helping patients survive it. The author, who has dedicated much of her career to learning about this topic and educating her peers on it, refers to these three key points as T3®.
Time
The first key point is making time to give time. Too often, our schedules are filled with pre-appointed hygiene procedures that a patient undergoes because of their insurance, and not necessarily because of their current disease presentation. Our dentists are occupied with repairing, rehabilitating, or surgically replacing dentition that has been underdiagnosed, undertreated, and ravished by pathogenic biofilm-or, if chronic in nature, referring the patient to a specialist for a more definitive diagnosis and invasive, costly treatment.
Our responsib ility, however, is to consider the patient's oral and systemic environment in detail, consider all risks associated with inflammation and its progression to overall health and wellness, record this information, and explain the consequences of noncompliance with the diagnoses and treatment options. This process is part of an optimized risk assessment protocol that is currently underutilized, resulting in poor risk management.
Data collection is essential to this process, yet very few offices are capable of scheduling sufficient time because they are booked out so far in advance with procedures. Lack of time prevents us from remembering or even using the tools available to increase efficiency, facilitate date-of-service diagnosis, and invoke co-diagnosis conversations with patients. Table 1 shows some resources the author utilizes to accomplish this.
Key Point:If we make the time to optimize these systems, we can give more efficient time in our schedules for assessment, education, and treatment so that disease resolution does not need to wait until the next available appointment.
Technology
According to a recent report, head and neck cancer accounts for 4% of all cancers in the US, with an estimated 15,050 deaths-mostly males-this year alone.1
Whilst numerous technological advances in prevention and screening are available, the author believes that they are underinvested in, underutilized, undertrained for, and in some ways feared. Part of the issue is time, as previously mentioned; most dental professionals still have not changed the way we schedule patients to include more definitive risk assessment protocols. New technology not taught in dental schools, regardless of graduation year, is adopted slowly if at all.
However, several visible fluorescence screening technologies utilize enhanced visualization of oral mucosal abnormalities. These bright blue light devices work by exciting and fluorescing normal soft tissue, making abnormal tissue appear darker to help the clinician see and document abnormalities not seen or felt with a comprehensive oral examination. Still, these devices do not diagnose cancer; only a pathologist can diagnose cancer with a sample of tissue. Dental hygienists simply have an opportunity to qualify these referrals much earlier.
One component contributing more to the incidence rate of oral cancers, particularly in the posterior, is human papillomavirus (HPV). Unfortunately, throat cancer has surpassed cervical cancer as the most common prevalent HPV infection-related cancer.2 The oropharynx, the tonsils, and the base of the tongue area are difficult to see with the naked eye, and because palpation examinations are subjective to time, technology, and training, many abnormalities go underdiagnosed.
Early oral HPV infections do not typically cause clinical signs or symptoms, so when any of the clinical signs of oral cancer are identified, they should be documented, photographed, and followed up on; additionally, confirming that the patient understands and hears what you are saying is critical. These clinical signs that should be investigated further include:
• Ulcer/sore that does not heal within 2 to 3 weeks
• Red, white, or black discoloration on soft tissues in the mouth
• Persistent sore throat or hoarse voice
• Odor or smell
• Difficult or painful swallowing
• Swelling or lump in the mouth
• Swollen but painless tonsil
• Pain when chewing
A simple saliva test can determine the presence of oral HPV in an active state.3,4 Unfortunately, a barrier to in-office laboratory testing is the fear of discussing costs that patients are not accustomed to encountering at a dental visit and that insurance will not cover. Dental professionals must overcome this barrier by explaining exactly what the test is, and they should ensure that the patient has considered all factors before making important health decisions based on an employer-sponsored discount plan that does not consider the stage, grade, toxicity, genetic, anatomical, identification, and management of collaborating factors.
High-risk HPVs (HR-HPVs) are responsible for the rising incidence of HPV-driven head and neck cancers, especially oropharyngeal cancers.3 Most HPV infections are asymptomatic and could clear within 2 years,5 but the antibodies still remain. A recent systematic review and meta-analysis determined that approximately 7.7% of healthy subjects harbor HPV in the oral region.6 A positive HPV test does not necessarily mean that oral cancer is present, but it does indicate an elevated risk with the need to monitor for signs and symptoms, and therefore increasing the availability of HPV tests is important, especially for patients who present with asymptomatic and hidden lesions. A patient presenting with a positive HPV test can be referred to an ear, nose, and throat specialist for an endoscopic examination and monitoring, because lesions often are in the back of the throat, at the base of the tongue, or within the folds of the tonsils-areas where dental professionals are limited in their ability to evaluate and document effectively and competently.
Screenings are preventive when calibrated and maximized by the clinician every time the patient presents for your care. For many years, the author has offered prevention therapy and biofilm care protocols through lectures and training programs, and it has become apparent that everyone does not see the same, examine the same, or articulate care the same. For this reason, the author has developed a synchronized training system for clinicians called SOSA©(Screening for Oral and Skin Abnormalities). The purposes of SOSA© are to synchronize screenings to save lives and to educate the community (including the public) about how self-examination of all skin surfaces, including the epithelium behind the lips, is important. SOSA© is not a cancer examination; instead, it involves looking for abnormalities in the oral cavity and soft skin areas that could potentially put the patient at risk for pain, inflammation, infection, or loss of teeth that ultimately changes the course of their life (Figure 3 and Figure 4).
Key Point:Take the TSA's advice: If you see something, say something, because you care. Educate yourself and your team on existing screening technologies and invest in time to be proficient, technology to facilitate comprehensive risk assessment and time management, and synchronized and calibrated training. Dental health providers who are skilled in providing intraoral and extraoral examination of soft and hard structures can save lives. Many clinicians still have a fear of discussing HPV and its role in cancer development, but preventing mortality and morbidity rates of late-stage diagnoses requires us to face that fear. Finally, we should not deprive the patient of the advancements of technology due to lacking an insurance code or adequate reimbursement. Come up with a plan. If you do not have a SOSA© protocol, the author is happy to share hers. Dispense tools necessary for your patients' wellbeing.
Training
When the whole team is able to articulate clear and concise explanations, the patient can understand the cause of disease and support the therapy planned to ensure their safety, health, form, and function. The combined efforts of the patient and the professional are required in order to manage the risk of disease long-term. Broadly speaking, this qualitative and quantitative process can be a combined effort to maximize the data attained in risk assessment and reduce any risk-related consequences by constantly tweaking the individual's potential risk management strategies.
Frequent team trainings, calibrated to competencies and incorporating scientifically proven technologies, can lower the number of people who develop disease and diagnose those with chronic disease before it could lead to lengthy and costly procedures or death.
Key Point: Empower the clinician to utilize necessary tools and technology for comprehensive risk assessment and time efficiency. The patient's medical history, the gift of open communication, and the art of listening all can make a positive impact. The dental profession prides itself on preventive care. Create a script if necessary to overcome the fear of rejection during the insurance chat. If you think you are creating an "unnecessary cost burden" to the patient, remind yourself and the patient of the potential costs of not screening, both financially and emotionally. Create a financial plan that can fit in the patient's budget or consider on a case-by-case basis absorbing the costs in order to have the peace of mind that you are doing what is necessary to provide the best assessment.
Discussion
The patient whose situation was described at the beginning of this article underwent several invasive procedures that involved utilizing tissue from his arm to create a flap-essentially, a new palate-after the surgical removal of the upper right maxilla and five teeth (Figure 5). Jeff was instructed to rest for a few weeks before beginning radiation. This phase was problematic as it created other challenges such as xerostomia, nausea, malaise, and financial burdens. Jeff credits his family and faith with propelling him forward. Today, he is healing and will endure the rigors of ongoing doctor visits, tests, and intensive, painful physical therapy to regain the range of motion in his neck and loss of strength in his shoulder and arm. In the future, he will contemplate restorative therapy to replace bone and implants to replace the teeth that were extracted and return his capabilities to properly masticate food.
The principles of T3 bring to mind a quotation attributed to Benjamin Franklin: "An ounce of prevention is worth a pound of cure."
In 2019, the national patient economic burden associated with cancer care was estimated to be $21.09 billion.7 This estimate includes patient out-of-pocket costs of $16.22 billion and patient time costs of $4.87 billion. Costs varied by age, stage at diagnosis, and phase of care. For example, out-of-pocket costs per person were generally higher among adult cancer survivors ages 18 to 64 than among those 65 or older.7,8Out-of-pocket costs were generally higher for patients diagnosed with later-stage disease and increase dramatically for end-of-life care.7
Scientists from the University of Pennsylvania's Perelman School of Medicine also found that a high out-of-pocket cost might be causing some patients to go without in-office visits as well. Half of all patients did not visit doctors regularly or pick up prescriptions when there was an out-of-pocket expense at or above $2,000. By comparison, only 10% failed to do so when out-of-pocket costs were lower than $10.7
Out-of-pocket costs include prescription drugs and medical services-such as provider and outpatient visits-emergency room visits, and hospital inpatient stays. For cancer survivors with health insurance coverage, out-of-pocket cost is their share of the cost that is not covered by insurance, such as copays, deductibles, and coinsurance. Cancer survivors without health insurance or with limited coverage may be responsible for the entire cost of care.8
The economic burden to patients with cancer is more than out-of-pocket costs. It also includes the time spent receiving medical care that could have been spent on a person's normal everyday activities. This part of the economic burden of cancer is called patient time cost.7 The time cost of cancer care (also called the opportunity cost) is the value of the time that cancer survivors spend traveling to and from care, waiting for care, and receiving care. It represents time not spent with friends and family, at work, or on leisure activities. Patient time costs are estimated by adding up the hours that a person with cancer usually spends on healthcare and multiplying it by the hourly value of patient time.7,8 The median wage rate for an adult is often used to represent this hourly value. Time costs can be substantial and can be an additional economic burden for cancer survivors and their families.
How does the investment in T3 compare to the cost in dollars to treat cancer? The researchers estimate that national costs for cancer-related medical services and oral prescription drugs in 2015 were $165 billion and $18 billion, respectively, totaling $183 billion. Based solely on population changes due to aging and growth, the researchers estimate that the national costs for cancer-related medical care and oral prescriptions drugs in 2030 will be $221 billion and $25 billion, respectively, totaling nearly $246 billion. This represents a total increase in national cost of 34%.8
Of course, it must be discussed how the COVID-19 pandemic will affect cancer statistics. Expect to see an increase in cancer mortality over the long term due to delayed diagnoses; interruptions or alterations in potentially curative treatment; the possibility that some adults will abandon prior patterns of preventive care; and the expectation that millions of adults will remain unemployed and without health insurance.9 The backlog of screening and other preventive healthcare visits will likely further exacerbate delayed diagnosis and substandard treatment among the underserved. The economic ramifications of the pandemic will only further widen this gap among individuals who were already financially insecure.10
In closing, the author would like to acknowledge how difficult this article was to write, articulating the profession's shortcomings in this area without expressing too much frustration or being offensive. This article is part of a drive and commitment to empower the profession to see the value in identifying imbalances in biofilm or abnormalities and their risk, before they become chronic and damage teeth or tissue, or cause cancer.
The patient whose case was discussed in this article is one of the author's closest friends. The news of his diagnosis triggered an emotional response that could have gone in a different direction had it not been for the author's conviction, training, and desire to support their journey. With the help and deep desire of friends in the industry, a package called KomfortKareKit© was compiled to address xerostomia and other oral symptoms so Jeff and other patients can focus on recovery and healing. Many of the products are listed in Table 1.
The author encourages any dental professionals to reach out for advice and guidance on this overall topic. Join us to make oral cancer history.
References
1. Head and Neck Cancer: Statistics. Cancer.Net website. https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics. Published February 2022. Accessed April 20, 2022.
2. Throat cancer now surpasses cervical cancer as the most common HPV-related cancer. Barbara Ann Karmanos Cancer Institute website. https://www.karmanos.org/karmanos/news/throat-cancer-now-surpasses-cervical-cancer-as-the-3289. Published April 1, 2021. Accessed April 20, 2022.
3. Weeramange CE et al. Salivary High-Risk Human Papillomavirus (HPV) DNA as a Biomarker for HPV-Driven Head and Neck Cancers. The Journal of Molecular Diagnostics. 2021;23(10):1334-1342.
4. Corstjens PLAM, Abrams WR, Malamud D. Detecting viruses by using salivary diagnostics. J Am Dent Assoc. 2012;143(10 Suppl):12S-18S.
5. Boda et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int J Oncol. 2018 Mar;52(3):637-655.
6. Betz SJ. HPV-Related Papillary Lesions of the Oral Mucosa: A Review. Head Neck Pathol. 2019;13(1):80-90.
7. Yabroff KR et al. Annual Report to the Nation on the Status of Cancer, Part 2: Patient Economic Burden Associated With Cancer Care. JNCI. 2021;113(12):1670-1682.
8. Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304-1312.
9. Shah ED, Amann ST, Karlitz JJ. The Time Is Now: A Guide to Sustainable Telemedicine During COVID-19 and Beyond. Am J Gastroenterol. 2020;115(9):1371-1375.
10. Balogun OD, Bea VJ, Phillips E. Disparities in Cancer Outcomes Due to COVID-19-A Tale of 2 Cities. JAMA Oncol. 2020;6(10):1531-1532.