You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Personal and professional peri-implant supportive care provides an environment that is favorable for long-term healthy retention of dental implants. Reasons for dental implant loss include bacterial infections, deosseointegration due to occlusal forces, and iatrogenic causes (eg, burning of bone during osteotomy creation, residual cement, etc).1 The most common clinical problems post implant insertion are peri-implant mucositis and peri-implantitis.2-4 Many articles address issues related to these two conditions with regard to their prevalence, incidence, risk factors, diagnostic findings, microbial data, influence of systemic disease, and treatment interventions.2-7 This article discusses why preventive care of dental implants is a critical determinant for reducing peri-implant diseases, maintaining peri-implant health, and enhancing the prognosis of dental implants.
Background Information: Implant Placements, Failure Rates, Infection
The American Academy of Implant Dentistry indicates that roughly 3 million people in the United States receive 5.5 million dental implants annually, and this quantity is projected to expand by 500,000 each forthcoming year.8 This increase in implant insertions is associated with more patients developing peri-implant mucositis and peri-implantitis.9 Thus, early detection and prevention of peri-implant diseases is an important aspect of clinical practice.
Dental implants do fail. About 2% to 3% of implants are lost prior to prosthetic loading, and another 2% to 3% that support fixed partial dentures are removed within 5 years.10 A systematic review retrospectively evaluated 10 long-term studies (>15 years) and noted that implant survival rates among the studies ranged from 70% to 100%. Eight of the 10 clinical trials indicated implant survival was >90%.11 Implant retention levels vary among studies, because there are differences related to the types of implants and their surfaces, treating clinician's experience, history of chronic periodontitis, bone quality, smoking status, and implant locations.12-14 While one can conclude that dental implant survival rates are high, these studies did not differentiate between success and survival of implants. Survival indicates that implants are present in the oral cavity, whereas success denotes that they are in function and exist in a state of health without bone loss.15,16
Experimental investigations in humans and animals have demonstrated development of peri-implant mucositis when implants are inserted and plaque control measures are stopped.17-20 Resumption of oral hygiene resolves the induced inflammation in 3 weeks or more.17,20These outcomes support the concept that peri-implant disease is bacterially induced. Risk factors (Table 1) can predispose and contribute to severity of the disease process.21-25 Other studies have indicated that when peri-implant mucositis is left untreated it can progress to peri-implantitis.22,26-30However, histopathologic and conversion conditions are not completely understood.22
The anatomy around teeth and dental implants is dissimilar. Teeth have gingival fibers that insert into cementum, whereas implants have parallel or oblique connective tissue fibers that adhere to the implant.31 Dental implants have less blood supply than teeth, because the periodontal ligament is absent.32 These anatomical differences may result in tissues surrounding dental implants being more susceptible to bacterial biofilm destruction than those surrounding teeth.33,34 Furthermore, once the body of the implant is accessible in the oral cavity, whether supra- or submarginally, removal of plaque is challenging because of the implant's macro-design and surface roughness.35 These facts highlight why patients who have received implants may require more frequent maintenance visits than typical dental patients who do not have dental implants.
Bacterial biofilms can induce an infection around dental implants, which may cause loss of osseous support, discomfort, and reduced levels of implant success.26 Because the word "infection" is used to describe a variety of conditions, several terms are defined to clarify intraoral situations. When there is an immune response to a bacterial challenge, it is considered an infection.36 Subclinical infections commonly occur in the crevice around teeth and dental implants. The term "subclinical" connotes that the patient's immune response has controlled the bacterial challenge around the implant and there are no clinical signs of an infection (eg, redness, swelling, etc).36 However, if the inflammatory process is not thwarted, tissue destruction occurs and the situation is considered a disease.36 For example, gingivitis and peri-implant mucositis, which histologically demonstrate an increased number of inflammatory cells and collagen-poor areas, are categorized as diseases.37,38 Similarly, periodontitis and peri-implantitis are diseases associated with chronic infections of the periodontium or peri-implant soft and hard tissues.22,39
Peri-implant Diseases: Definitions and Prevalence Rates
According to the new periodontal classification scheme,peri-implant diseases are classified as follows:
Peri-implant mucositis. This condition usually manifests bleeding on probing (BOP) with visual signs of inflammation and no bone loss. Robust evidence shows that peri-implant mucositis is caused by plaque and can be resolved with treatment directed at removing plaque.21
Peri-implantitis. Peri-implantitis is a plaque-induced pathologic disorder of tissues surrounding a dental implant; there is inflammation in the peri-implant mucosa and loss of supportive bone that may be progressive.22 Peri-implant mucositis is presumed to precede peri-implantitis,27-30,40and peri-implantitis is often found among patients with a history of periodontitis.22,41-43The onset of peri-implantitis may occur early after implant placement.44 If left untreated, peri-implantitis appears to proceed in a non-linear and accelerating pattern of bone destruction.22 With respect to the chronology of peri-implantitis development, signs of bone loss have been detected by 18 to 24 months45 or after the second or third year post implant insertion44 but can appear at an earlier time point.
Clinicians generally are aware of the high survival rates of dental implants but may not be alert to the vast incidence of peri-implant mucositis and peri-implantitis subsequent to implantations. The finding of elevated rates of peri-implant diseases is not surprising considering the observation that 74% of implants in partially edentulous patients had inadequate plaque control by patients.46 Several systematic reviews documented the prevalence of peri-implant mucositis and peri-implantitis with respect to the number of patients and the quantity of dental implants that experience disease occurrence.3,4,7 Atieh et al included nine studies with 1,497 participants and 6,283 implants in their review.3 Criteria for peri-implantitis were BOP, probing depth (PD) ≥5 mm, cumulative bone loss ≥2 mm or three or more exposed threads, and the implants were observed ≥5 years. They reported that peri-implant mucositis prevalence among patients was 63.4% and 30.7% with respect to implants. Also, 18.8% of patients and 9.6% of implants developed peri-implantitis.3 Note that the percentage of patients is always higher than the proportion of implants involved, because a patient can have multiple implants unaffected. Furthermore, percentages varied among studies, because the criteria (PD and/or quantity of bone loss) used to define peri-implantitis may differ.
In another systematic review that included 47 studies of at least 3 years duration, Lee et al reported that mean patient and implant prevalences for peri-implant mucositis were, respectively, 46.83% and 29.48%. With regard to subject- and implant-based peri-implantitis, the occurrences were, respectively, 19.83% and 9.25%.4 At the 11th European Workshop in Periodontology consensus conference in 2014, at which 11 studies were evaluated, a mean patient prevalence rate of 43% was noted for peri-implant mucositis and 23% for peri-implantitis after implants were placed.7
These studies indicate that despite the relatively high survival rate of dental implants, complicating inflammatory processes often compromise implant health.3,4,7
Criteria Used to Define Peri-implantitis Affects Prevalence Rates
A systematic review assessed 32 studies to determine how different clinical criteria (ie, BOP and PD) used to evaluate the level of peri-implantitis affected disease prevalence.47 Overall, it was ascertained that 17% of subjects and 11% of implants had peri-implantitis. However, when the criterion to define peri-implantitis with respect to probing depth was altered, its occurrence changed. For example, if the threshold PD for peri-implantitis was ≥4 mm, the subject and implant peri-implantitis prevalences were, respectively, 34% and 11%; if PD ≥5 mm was employed, the subject and implant levels of peri-implantitis dropped to 12% and 10%, respectively.
Thus, the sensitivity to assess prevalence of peri-implantitis is affected by the criteria used to define the disease, and the overall true prevalence may currently be incorrectly estimated. Desirable probing depths around dental implants are 2.5 mm to 4 mm, but deeper assessments can be associated with healthy peri-implant mucosa.48 Therefore, at probing depths ≥4 mm there is an assumption that supporting bone has been lost; however, this PD may not reflect a pocket as sometimes implants are placed very submucosally because the tissue is thick, and this results in increased peri-implant probing depths.
Value of Clinical Parameters to Detect Peri-implantitis
Bleeding on probing around individual implants or among patients with implants was able to identify only 24.1% of implant sites with peri-implantitis and 33.8% of patients with peri-implantitis (some patients have more than one implant).49 Therefore, bleeding is not a good identifier of sites or patients with peri-implantitis. However, the absence of BOP is a good indicator for a stable peri-implant condition with respect to future bone loss.26 The false-positive rate of BOP to diagnose peri-implantitis underscores the need to probe patients at maintenance visits50 and to periodically obtain radiographs (at least every 2 years) to assess for bone level alterations.51After an implant is placed, bone remodeling always occurs adjacent to bone-level implants (1 mm to 2 mm)52 and to a lesser degree adjacent to tissue-level implants (0.75 mm to 0.85 mm)53; this occurs to accommodate biologic width formation (epithelial and connective tissue adherence). Therefore, baseline bone level assessments should not be recorded until after restoration placement or several months after placement of an abutment.52
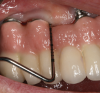
Probing as a diagnostic indicator of peri-implant disease is technique sensitive and should be done with a light probing force that is less than what is usually used around teeth (eg, force with a probe that blanches a nail bed).54 Probing evaluations are important because they can detect probing depth alterations, which may reflect peri-implant pathosis. Clinicians should note that restorative designs that limit access to the peri-implant crevice can complicate probing assessments (Figure 1).
Effect of Professional Maintenance on Peri-implant Diseases
Numerous studies have indicated that periodontal maintenance helps reduce the occurrence of peri-implantitis.3,5,24,55,56 In Table 2, investigations are listed that demonstrated reduced levels of peri-implantitis among patients who have regular supportive therapy compared to individuals who do not have regular peri-implant maintenance.3,5,24,55-61 Several articles, as noted in the following paragraphs, have demonstrated specific benefits provided by peri-implant supportive therapy.
Rinke et al reported regular supportive therapy significantly decreases the risk for peri-implantitis compared with irregular maintenance.60 Their data indicated that individuals who do not engage in regular post-treatment maintenance have an 11-fold greater chance to experience peri-implantitis than patients with good compliance. The odds ratio for developing peri-implantitis with regular maintenance was 0.09.
Quirynen et al noted (in a review that comprised 16 studies) that patients with a history of periodontitis who had moderately rough-surfaced implants can successfully function long term when there is regular supportive periodontal therapy.62 Patients with scheduled maintenance visits manifested fewer peri-implant issues than patients without regular supportive therapy.
Gay et al assessed more than 1,000 implant patients over 17 years and reported that individuals without maintenance had a lower survival rate than patients with supportive therapy.58 Patients with regular maintenance (at least one visit per year) had implant loss rate reduced by 90%. This study did not account for the effect of smoking or prosthesis design.
Finally, Ramanauskaite and Tervonen reported that in all investigations (12 in a systematic review) where there was a lack of peri-implant maintenance, an increased number of sites manifested mucosal bleeding, deepened pockets, or alveolar bone loss and increased implant loss.29
It can be concluded that although peri-implant supportive therapy may not necessarily help all patients, when the data is examined cumulatively, it is apparent that compared to individuals who do not undergo supportive therapy, many patients who receive peri-implant maintenance have better results with respect to reduced levels of peri-implantitis,3,5,30,57 less bone loss,30,55,57 fewer deep pockets,55,56 and less implant loss.55,58,60,63
Maintenance Appointment Frequency; Effect of Periodontitis History
It is well established that maintenance visits help reduce the incidence of peri-implant diseases (Table 2).3,5,24,55-61 Suggested professional recall intervals are listed in Table 3.29,47,51,55,58-60,63-69However, with respect to implant maintenance these intervals are based on opinion, because no clinical trial has assessed the efficacy of different supportive therapy intervals. Furthermore, no one recall interval is correct for all patients.
Nowzari and Jorgensen advised that other issues besides bacterial control need to be considered when deciding maintenance intervals.67 Such issues include periodontitis and/or peri-implantitis history, efficacy of daily plaque control, smoking, rate of calculus formation, peri-implant BOP and/or suppuration, and peri-implant probing depths and radiographic bone loss. Similarly, a consensus report from a meeting of 25 experts concluded that supportive therapy intervals and protocols must be individually determined based on the patient's original condition and risk factors.51 They suggested that maintenance intervals should have a specific periodicity (eg, every 3 to 4 months). Since there is no controlled clinical trial assessing the efficacy of different recall intervals, expert opinion based on experience is the best evidence available at this time. It should also be noted, however, that expert opinion is considered category D level of evidence, which is a low level of evidence (usually scored A to D).69
Patients with a history of periodontitis are more susceptible to developing peri-implantitis than individuals who never had periodontitis.2,6,22,41-43 This has several implications. It is imperative that patients with a periodontitis history who receive implants be maintained and monitored carefully to reduce development of peri-implantitis. Their recall interval should reflect what is needed to sustain periodontal as well as peri-implant health. Patients with periodontal issues, such as deep probing depths around unhealthy teeth, have an increased risk of developing peri-implantitis.6 In this regard, Pjetursson et al reported that in periodontitis-susceptible patients, residual pockets (PD ≥5 mm) at the end of active periodontal therapy represent a significant risk for the development of peri-implantitis and implant loss.6 Furthermore, reports have cautioned against inserting implants into a periodontally unhealthy mouth, because implant sites can be initially colonized by bacteria that may be unfavorable to peri-implant health.70,71
Measures to Control Peri-implant Mucositis and Peri-implantitis
Numerous articles have addressed various techniques that can be used by patients and therapists to control plaque and calculus around dental implants.64-67,72-80 Some basic salient points, as discussed in the following paragraphs, are worth noting.
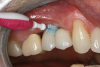
Personal hygiene aids: manual and electric toothbrushes, dental floss, interproximal brushes, and antimicrobials. For single implants or fixed multiunit implant-supported prostheses, plaque deposits may be managed using a manual or powered toothbrush with floss or an interdental brush.67 Interproximally, interdental brushes with plastic coated wire reduce scratching of implant surfaces (Figure 2).80 Floss threaders are an important aid for use with multiunit prostheses.72 Subgingival irrigation with or without antimicrobials (eg, chlorhexidine) can also be used as an adjunct to reduce plaque deposits.75,76,81 Patients should be instructed to brush implants twice daily to reduce bacterial plaque accumulations.64
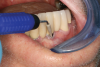
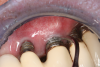
Professional maintenance devices: scalers (manual, sonic, ultrasonic) and polishers.Clinicians performing deplaquing or calculus removal from an implant-supported restoration need to be aware they may possibly engage different types of surfaces, eg, restoration, smooth abutment, rough implant surface. To preclude roughening an abutment surface, a plastic instrument could be employed manually, even though residues from the instrument may be left behind.77 Metal sonic and ultrasonic instruments can be used when scaling abutments; however, to avoid micro-roughening of abutments with these instruments, nylon sleeves and plastic inserts can be utilized (Figure 3).78,79 Because plastic instruments are not effective in debriding rough-surface implants, metal hand or ultrasonic instrumentation can be used to clean an implant body that is inherently rough (Figure 4).82,83 The surfaces of titanium implants can be polished using a rubber cup with a non-abrasive paste.65 Finally, the patient's informed consent form should include the responsibility to be compliant with personal and professional peri-implant maintenance.
Conclusions
Clinicians need to be acutely aware that the prevalence rates of peri-implant mucositis and peri-implantitis post dental implantations are very high. Personal and professional peri-implant maintenance therapy after dental implant insertion provides the best results for the most patients with respect to reducing the occurrence of peri-implant mucositis and peri-implantitis. The frequency of recall intervals needs to be tailored to the individual, taking into account numerous risk factors and the reliability of the patient to perform personal oral hygiene. Patients need to be informed of the importance of professional and personal hygiene. This concept should be incorporated into patients' informed consent to underscore that their cooperation is needed to enhance success of their dental implantations.
About the Authors
Gary Greenstein, DDS, MS
Former Clinical Professor, Division of Periodontics, Section of Oral, Diagnostic, and Rehabilitation Sciences, College of Dental Medicine, Columbia University, New York, New York; Private Practice, limited to Periodontics and Dental Implantology, Freehold, New Jersey
Robert Eskow, DMD, MScD
Clinical Professor, Division of Periodontics, Section of Oral, Diagnostic, and Rehabilitation Sciences, College of Dental Medicine, Columbia University, New York, New York; Private Practice limited to Periodontics, Dental Implantology, and Oral Medicine, Livingston and Clark, New Jersey
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Esposito M, Thomsen P, Ericson L, Lekholm U. Histopathologic observations on early oral implant failures. Int J Oral Maxillofac Implants. 1999;14(6):798-810.
2. Ting M, Craig J, Balkin BE, Suzuki JB. Peri-implantitis: a comprehensive overview of systematic reviews. J Oral Implantol. 2018:44(3);225-247.
3. Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84(11):1586-1598.
4. Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1-12.
5. Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017;88(10):1030-1041.
6. Pjetursson BE, Helbling C, Weber HP, et al. Peri-implantitis susceptibility as it relates to periodontal therapy and supportive care [erratum appears in Clin Oral Implants Res. 2012;23(8):1004]. Clin Oral Implants Res. 2012;23(7):888-894.
7. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(suppl 16):S158-S171.
8. Market Analysis Report. Dental Implants Market Size, Share & Trends Analysis Report By Implants Type (Titanium, Zirconium), By Region (North America, Europe, Asia Pacific, Latin America, MEA), And Segment Forecasts, 2021 - 2028. San Francisco, CA: Grand View Research; February 2021. https://www.grandviewresearch.com/industry-analysis/dental-implants-market#:~:text=The%20U.S.%20held%20a%20substantial,per%20the%20American%20Dental%20Association. Accessed February 21, 2022.
9. Valente NA, Andreana S. Peri-implant disease: what we know and what we need to know. J Periodontal Implant Sci. 2016;46(3):136-151.
10. Holm-Pedersen P, Lang NP, Müller F. What are the longevities of teeth and oral implants? Clin Oral Implants Res. 2007;18(suppl 3):15-19.
11. Levin L, Halperin-Sternfeld M. Tooth preservation or implant placement: a systematic review of long-term tooth and implant survival rates. J Am Dent Assoc. 2013;144(10):1119-1133.
12. Alsaadi G, Quirynen M, van Steenberghe D. The importance of implant surface characteristics in the replacement of failed implants. Int J Oral Maxillofac Implants. 2006;21(2):270-274.
13. Alsaadi G, Quiryen M, Michiles K, et al. Impact of local and systemic factors on the incidence of implant failures, up to abutment connection with modified surface oral implants. J Clin Periodontol. 2008;35(1):51-57.
14. Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34(7):610-617.
15. Setzer FC, Kim S. Comparison of long-term survival of implants and endodontically treated teeth. J Dent Res. 2014;93(1):19-26.
16. Papaspyridakos P, Chen CJ, Singh M, et al. Success criteria in implant dentistry: a systematic review. J Dent Res. 2012;91(3):242-248.
17. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-259.
18. Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28(6):517-523.
19. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
20. Meyer S, Giannopoulou C, Courvoisier D, et al. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2017;28(8):1005-1012.
21. Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Periodontol. 2018;89(suppl 1):S257-S266.
22. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(suppl 1):S267-S290.
23. Kordbacheh Changi K, Finkelstein J, Papapanou PN. Peri-implantitis prevalence, incidence rate, and risk factors: a study of electronic health records at a U.S. dental school. Clin Oral Implants Res. 2019;30(4):306-314.
24. Dreyer H, Grischke J, Tiede C, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res. 2018;53(5):657-681.
25. Hashim D, Cionca N. A comprehensive review of peri-implantitis risk factors. Curr Oral Health Rep. 2020;7:262-273.
26. Jepsen S, Rühling A, Jepsen K, et al. Progressive peri-implantitis. Incidence and prediction of peri-implant attachment loss. Clin Oral Implants Res. 1996;7(2):133-142.
27. Zeza B, Pilloni A. Peri-implant mucositis treatments in humans: a systematic review. Ann Stomatol (Roma). 2012;3(3-4):83-89.
28. Albouy JP, Abrahamsson I, Persson LG, Berglundh T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: histological observations. Clin Oral Implants Res. 2009;20(4):366-371.
29. Ramanauskaite A, Tervonen T. The efficacy of supportive peri-implant therapies in preventing peri-implantitis and implant loss: a systematic review of the literature. J Oral Maxillofac Res. 2016;7(3):e12.
30. Costa FO, Takenaka-Martinez S, Cota LO, et al. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39(2):173-181.
31. Berglundh T, Lindhe J, Ericsson I, et al. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2(2):81-90.
32. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21(3):189-193.
33. Ericsson I, Berglundh T, Marinello C, et al. Long-standing plaque and gingivitis at implants and teeth in the dog. Clin Oral Implants Res. 1992;3(3):99-103.
34. Lindhe J, Berglundh T, Ericsson I, et al. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3(1):9-16.
35. Gomes SC, Corvello P, Romagna R, et al. How do peri-implant mucositis and gingivitis respond to supragingival biofilm control - an intra-individual longitudinal cohort study. Eur J Oral Implantol. 2015;8(1):65-73.
36. Evans AS. Epidemiological concepts. In: Evans AS, Brachmen PS, eds. Bacterial Infections of Humans: Epidemiology and Control. New York, NY: Plenum Press; 1991:3-58.
37. Greenstein G, Caton J, Polson AM. Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol. 1981;52(8):420-425.
38. Khammissa RA, Feller L, Meyerov R, Lemmer J. Peri-implant mucositis and peri-implantitis: clinical and histopathological characteristics and treatment. SADJ. 2012;67(3):122,124-126.
39. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(suppl 1):S173-S182.
40. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol. 2018;89(suppl 1):S1-S8.
41. Ferreira SD, Silva GL, Cortelli JR, et al. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33(12):929-935.
42. Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33(4):290-295.
43. Karoussis IK, Salvi GE, Heitz-Mayfield LJ, et al. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003;14(3):329-339.
44. Derks J, Schaller D, Håkansson J, et al. Peri-implantitis - onset and pattern of progression. J Clin Periodontol. 2016;43(4):383-388.
45. Becker J, John G, Becker K, et al. Clinical performance of two-piece zirconia implants in the posterior mandible and maxilla: a prospective cohort study over 2 years. Clin Oral Implants Res. 2017;28(1):29-35.
46. Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20(2):169-174.
47. Muñoz V, Duque A, Giraldo A, Manrique R. Prevalence of peri-implant disease according to periodontal probing depth and bleeding on probing: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33(4):e89-e105.
48. Dhir S, Mahesh L, Kurtzman GM, Vandana KL. Peri-implant and periodontal tissues: a review of differences and similarities. Compend Contin Educ Dent. 2013;34(7):e69-e75.
49. Hashim D, Cionca N, Combescure C, Mombelli A. The diagnosis of peri-implantitis: a systematic review on the predictive value of bleeding on probing. Clin Oral Implants Res. 2018;29(suppl 16):276-293.
50. Tarnow DP. An interview with Dr. Dennis Tarnow: the propensity for probing around dental implants. Implant Dent. 2018;27(2):151-152.
51. Del Fabbro M, Nevins M, Venturoli D, et al. Clinically oriented patient maintenance protocol: a clinical consensus of experts. Int J Periodontics Restorative Dent. 2018;38(2):281-288.
52. Cochran DL, Hermann JS, Schenk RK, et al. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997;68(2):186-198.
53. Brägger U, Häfeli U, Huber B, et al. Evaluation of postsurgical crestal bone levels adjacent to non-submerged dental implants. Clin Oral Implants Res. 1998;9(4):218-224.
54. Gerber JA, Tan WC, Balmer TE, et al. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin Oral Implants Res. 2009;20(1):75-78.
55. Roccuzzo M, De Angelis N, Bonino L, Aglietta M. Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: implant loss and radiographic bone loss. Clin Oral Implants Res. 2010;21(5):490-496.
56. Roccuzzo M, Bonino F, Aglietta M, Dalmasso P. Ten-year results of a three arms prospective cohort study on implants in periodontally compromised patients. Part 2: clinical results. Clin Oral Implants Res. 2012;23(4):389-395.
57. Zangrando MS, Damante CA, Sant'Ana AC, et al. Long-term evaluation of periodontal parameters and implant outcomes in periodontally compromised patients: a systematic review. J Periodontol. 2015;86(2):201-221.
58. Gay IC, Tran DT, Weltman R, et al. Role of supportive maintenance therapy on implant survival: a university-based 17 years retrospective analysis. Int J Dent Hyg. 2016;14(4):267-271.
59. Aguirre-Zorzano LA, Vallejo-Aisa FJ, Estefania-Fresco R. Supportive periodontal therapy and periodontal biotype as prognostic factors in implants placed in patients with a history of periodontitis. Med Oral Patol Oral Cir Bucal. 2013;18(5):786-792.
60. Rinke S, Ohl S, Ziebolz, D, et al. Prevalence of periimplant disease in partially edentulous patients: a practice-based cross-sectional study. Clin Oral Implants Res. 2011;22(8):826-833.
61. Lin CY, Chen Z, Pan WL, Wang HL. The effect of supportive care in preventing peri-implant diseases and implant loss: a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(8):714-724.
62. Quirynen M, Abarca M, Van Assche N, et al. Impact of supportive periodontal therapy and implant surface roughness on implant outcome in patients with a history of periodontitis. J Clin Periodontol. 2007;34(9):805-815.
63. Anner R, Grossmann Y, Anner Y, Levin L. Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival: a long-term retrospective evaluation of patients followed for up to 10 years. Implant Dent. 2010;19(1):57-64.
64. Gulati M, Govila V, Anand V, Anand B. Implant maintenance: a clinical update. Int Sch Res Notices. 2014;2014:908534.
65. Kanathila H, Pangi A, Benakatti V, Patil S. Maintenance of dental implants: a way to long term success - a review. Int J Applied Dent Sci. 2018;4(2):104-107.
66. Schmage P, Kahili F, Nergiz I, et al. Cleaning effectiveness of implant prophylaxis instruments. Int J Oral Maxillofac Implants.2014;29(2):331-337.
67. Nowzari H, Jorgensen M. Dental implant maintenance. In: Principles and Practice of Single Implant and Restorations. Torabinejad M, Sabeti MA, Goodacre CJ, eds. Elsevier Saunders; 2014.
68. Howe MS. Implant maintenance treatment and peri-implant health. Evid Based Dent. 2017;18(1):8-10.
69. Bidra AS, Daubert DM, Garcia LT, et al. Clinical practice guidelines for recall and maintenance of patients with tooth-borne and implant-borne dental restorations. J Am Dent Assoc. 2016;147(1):67-74.
70. Danser MM, van Winkelhoff AJ, van der Velden U. Periodontal bacteria colonizing oral mucous membranes in edentulous patients wearing dental implants. J Periodontol. 1997;68(3):209-216.
71. Quirynen M, Vogels R, Peeters W, et al. Dynamics of initial subgingival colonization of ‘pristine' peri-implant pockets. Clin Oral Implants Res. 2006;17(1):25-37.
72. Eskow RN, Smith VS. Preventive periimplant protocol. Compend Contin Educ Dent. 1999;20(2):137-146.
73. Sternberg-Smith V, Eskow RN, Tarnow DP. Assessment is the key to implant success. Dimensions of Dental Hygiene. 2005;3(7):14-16.
74. Hatzimanolakis P, Tsourounakis I, Kelekis-Cholakis A. Dental implant maintenance for the oral healthcare team. Compend Contin Educ Dent. 2019;40(7):424-429.
75. Jolkovsky DL, Lyle DM. Safety of a water flosser: a literature review. Compend Contin Educ Dent. 2015;36(2):146-149.
76. Lyle DM. Use of a water flosser for interdental cleaning. Compend Contin Educ Dent. 2011;32(9):78-82.
77. Humphrey S. Implant maintenance. Dent Clin North Am. 2006;50
(3):463-478.
78. Silverstein LH, Kurtzman GM. Oral hygiene and maintenance of dental implants. Dent Today. 2006;25(3):70-75.
79. Patil SH, Veena HR, Mahantesha C, et al. Dental implant maintenance - a review. Int J Dental Clinics. 2012;4(1):37-40.
80. Hwang JW. Practical implant dentistry. J Prosthodontics. 2006;15
(3):214-216.
81. Mayer Y, Ginesin O, Horwitz J. A nonsurgical treatment of peri-implantitis using mechanic, antiseptic and anti-inflammatory treatment: 1 year follow-up. Clin Exp Dent Res. 2020;6(4):478-485.
82. Park JB, Jang YJ, Koh M, et al. In vitro analysis of the efficacy of ultrasonic scalers and a toothbrush for removing bacteria from resorbable blast material titanium disks. J Periodontol. 2013;84(8):1191-1198.
83. Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009;80(11):1824-1832.