You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Despite multiple prevention strategies, data from both the National Institutes of Health and Centers for Disease Control and Prevention show that caries and periodontal disease prevalence for adults in the United States are still at all-time highs (92% and 47%, respectively).1,2 With the introduction of nanotechnology-ie, technology involving dimensions of less than 100 nanometers (nm), particularly the manipulation of individual atoms and molecules-new materials have become available to help address a variety of oral care issues, opening the door for new prevention strategies.
A most interesting and useful nanomaterial being studied in oral care is silver nanoparticles (AgNPs), which have been shown to have unique antimicrobial, antifungal, and antiviral properties.3 In medicine, silver nanoparticles have been used successfully in many applications ranging from heart valves to wound care.4 Dentistry has also started incorporating silver nanoparticles into practice for their many potential uses. This article will explore the preventative and restorative applications of AgNPs.
What Are Silver Nanoparticles (AgNPs)?
AgNPs are small conglomerations of reduced silver atoms compacted into a single unit. They can range anywhere from 1 nm to 100 nm. A transmission electron microscopy(TEM) of plant-based AgNPs is shown in Figure 1. Typically, the most studied types of nanoparticles have a size range between 5 nm and 50 nm. This is because this size profile is uniquely advantageous against microbial pathogens.5 AgNPs can be manufactured with a variety of custom coatings and be made into different sizes and shapes depending on the application. This process allows for far more versatility than traditional antimicrobials. As a result, AgNPs have been used in a wide variety of medical applications from eye treatments to catheters and other medical devices.6
Synthesis Methods and Toxicity
There are three distinct methods for the production of AgNPs. These methods are broadly classified as physical, chemical, or biological methods.7 The physical method for synthesis of AgNPs is the oldest method and is still commonly used; this can be done using electrolysis or lasers. This process can be extremely energy intensive and yields inconsistent particle sizes.8 Unlike with newer methods, the particles resulting from this process also lack a coating. This can result in less-than-desirable outputs, leading to instability in various environments outside of deionized water.
The second-generation synthesis process, commonly referred to as the chemical method, has also seen wide use in multiple industries. Although this method improved upon size consistency profiles, these particles tend to have higher toxicity profiles. This is because strong reducing agents are required for the synthesis process.9 Chemically reduced particles also tend to be more unstable in high ionic strength environments, which are common in saliva, blood, and various tissue types.10
It also is notable that the physical and chemical methods of producing AgNPs have a propensity for staining at high concentrations. This is due to lower stability in various environments, which causes faster release of silver ions.11 This material shortcoming has limited the use of AgNPs in dentistry, especially in resin matrices.12 These effects also carry over to environmental concerns regarding toxicity, which have been the subject of debate for some time. Recent advancements in biological reduction pathways for AgNPs, however, have shown increased stability and have reduced environmental concerns.13
In an effort to overcome the deficiencies of the earlier AgNP production processes, scientists have started exploring third-generation biological plant-based reduction methods. Although this science is more recent, work has shown that these particles have increased biocompatibility and higher-stability profiles in a variety of in vivo and high ionic strength environments.14
Mechanism of Action of AgNPs
AgNPs have been extensively studied for decades. Their primary mechanism of action is the adherence to and penetration into bacterial cells.15 AgNPs also can release silver ions locally, which subsequently latch onto bacteria, causing cellular dysfunction and rupture.15 The large charge-to-size profiles of AgNPs can ultimately lead to dysfunction of the membrane potential of bacterial cells when in close proximity.15 Although their mechanism of action is effective against many microbial pathogens, biologically synthesized AgNPs, in particular, have exhibited very low toxic profiles on mammalian cells.16
AgNPs as Antimicrobials
Many studies have been done on the preventative aspects of silver nanoparticles. One of the most interesting properties of these small particles is their ability to kill oral pathogens. AgNPs have been shown to have anti-adherence properties, as well as effects on shifting oral microenvironments away from pathogenesis.17
Quaternary ammonium compounds (QACs) such as chlorhexidine (CHX) and cetylpyridinium chloride (CPC) have undesirable side-effect profiles, including staining, allergic reactions, taste changes, and increased risk for calculus buildup.18 Additionally, concerns have been raised about the toxic profiles of QACs on tissue, such as odontoblasts, fibroblasts, and periodontal ligament cells.19,20 Conversely, antimicrobial effects of AgNPs have been shown to be as low as 10 ppm, or approximately 120 times lower than the concentration of CHX.21 In a recent study, AgNPs have even shown comparable anti-gingivitis effects to CHX over a 6-week period.22 Recent work on periodontal pockets has shown that AgNPs also can elicit reduction in pocket depths when applied as a gel or rinse, and these applications were shown to be comparable to tetracycline applications.23
Microscopic pre- and post-treatment images of plaque from a periodontal pocket treated with AgNPs are shown in Figure 2 and Figure 3.
The "Zombies Effect"
A phenomenon known as the "zombies effect" describes how bacteria that have died from exposure to AgNPs can become carriers of silver nanoparticles.24 Once these bacterial cells die they begin to increasingly soak up AgNPs until they reach a plateau of silver concentration. When this occurs, the affected bacteria become net releasers of silver nanoparticles, causing adjacent bacteria to die. Le-Chatelier's principle dictates the release of these AgNPs from zombie cells, providing an extended antimicrobial effect.24 This can ultimately continue the destruction of various pathogenic bacteria, and is a unique effect of AgNPs. As a result, further bacterial growth at wound sites can be reduced, promoting the healing process.
Disruption of Oral Biofilms
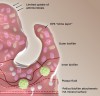
In the oral microenvironment, one of the greatest challenges for antimicrobials has been the penetration and disruption of biofilms.25 A cut-away view of an oral biofilm is shown in Figure 4. One of the least understood aspects of oral biofilms has been their ability to prevent entry of charged antimicrobials and remineralization agents. The outer layer of plaque consists of an exopolysaccharide matrix. This matrix, which is produced by biofilms, can impede antimicrobial agents and prevent bioactives from reaching their intended target sites.25 As a result, plaque fluid, ie, deeper acidic microenvironments near the tooth/plaque interface, remains untouched. Ultimately this can lead to extended periods of demineralization and subsequent caries.
A recent study showed how remineralizing agents can be used synergistically with AgNPs to increase their uptake into plaque.26 As a result, AgNPs can be utilized as a delivery system for various remineralization compounds that when used alone exhibit low penetration potential into plaque.26 By using AgNPs in combination with other agents, plaque acids can be quickly neutralized and pathogenesis can be mitigated.
AgNPs in Caries Control
Caries control strategies have long been dominated by fluoridated agents, but it has become apparent that fluoride's effectiveness can be hampered by lack of delivery into plaque fluid.27 A study showed that a 1000 ppm fluoride rinse may deliver only less than 60 ppm directly into the inner plaque mass, with this effect decreasing over time as the biofilm matures.27 Fluoride's anti-acidogenic properties have been documented to be optimal at 190 ppm, suggesting the need for improved delivery systems to penetrate plaque.28 Poor delivery into the deeper portions of plaque has also been documented for other charged agents such as calcium and sodium bicarbonate. This can prevent the neutralization of acidogenic species from beneficial agents.26
Therefore, AgNPs have been suggested as a carrier system for use alongside remineralizing agents due to their superior penetration potential into plaque as well as their anti-acidogenic properties on Streptococcus mutans.26,29 Recent work has shown AgNPs with fluoride (also known as nanosilver fluoride) and with calcium to have advantageous synergistic potential over the use of these remineralizing agents alone.26,30
AgNPs as Remineralization Agents
AgNPs have been a topic of interest in the realm of remineralization strategies, as several studies showed them to improve the microhardness of teeth without the use of fluoride. One recent study showed increased microhardness when AgNPs were used; specifically, AgNPs were shown to increase the microhardness of enamel samples over controls by 15%.29 Another study showed that AgNPs can be used as delivery systems for remineralization agents and, thus, act indirectly to increase the microhardness of hydroxyapatite (HA).26 Although more research is necessary, it is clear that silver atoms can deposit into damaged HA sites, eliciting a hardening effect.31 Cation contribution to remineralization has confirmed that certain metals will ultimately contribute to increased microhardness of HA, as has been shown with silver diamine fluoride, stannous fluoride, and other preventative agents.31,32
Prevention of Demineralization
AgNPs have been shown to be highly effective at preventing demineralization. Utilized in orthodontic bracket cement and sealants, silver nanoparticles showed significant reductions in demineralization around high-risk areas compared to controls without them.33 Some studies have even shown the anti-cariogenic effects of these materials through release of calcium while resisting oral biofilms simultaneously.34 This is because the small size and alkaline pH of AgNPs can alter acidic biofilms effectively and, thus, control acid release directly within plaque.26,34
Anti-inflammatory Effects
Inflammation is an ongoing process that is entrenched in many oral diseases. AgNPs have shown great promise for reduction of inflammation. Specifically, tests done in vivo have shown pocket depth reductions as well as anti-gingivitis effects.22,23 AgNPs have been tested against CHX in vivo and shown comparable results, with little to no side effects.22 Additionally, studies done with AgNPs have shown that surgical sites healed much faster with less hypertrophy and scarring.35 These materials are also being tested as implant coatings and have shown superior results regarding bacterial attachment compared to titanium surfaces alone.36
Custom Coating and Biomimicry
In the past, AgNPs were made primarily without coatings. With today's materials, AgNPs can be modified with a variety of coatings, such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), polysaccharides, and plant leaf extracts. These biological coatings have been used in drug delivery systems, solar cells, and for imaging systems.37 By selecting a custom coating, it is now possible to mimic similar surfaces employed by biofilms. This is due to the fact that plant-based polysaccharides closely match the chemical structure of a biofilm's outer extracellular polymeric substances (EPS) layer.26 The EPS layer has long presented a clinical challenge as it protects biofilms from antimicrobials and antibiotics.25 With nanoengineering, however, it is now possible to create a situation where biofilms can no longer effectively distinguish AgNPs from their own EPS coatings. In theory, these types of systems could revolutionize biofilm management, although more research is needed.
AgNPs in Restorative Materials
The advent of nanotechnology has also impacted the development of restorative materials. Many universities have begun testing and publishing the use of silver nanoparticles in re-wetting agents, sealants, composites, cements, and gutta-percha. This is because AgNPs have the ability to deter bacterial microleakage and adherence, and reduce matrix metalloproteinase (MMP) interactions in bonded restorations.12,34
AgNPs have been appreciably discussed in the literature for their use in bonding systems. These materials have shown that they can be utilized to reduce bacterial biofilm attachment and microleakage in composite restorations.34 Ultimately, AgNPs show great promise for incorporation into bonding protocols and will likely be used more frequently in dentistry in due time because of their high retention of bond strength via MMP deactivation and antimicrobial action.12,34
Conclusion
As dentistry progresses, new and better materials can be expected to emerge. Although there were challenges with previous attempts to incorporate AgNPs into materials, as with the first- and second-generation production processes, new and exciting breakthroughs are changing how these materials are produced and used. AgNPs have been shown to be extremely effective against biofilms,25 as carrier systems,26 and as anti-caries agents.29,34 Newer-generation plant-based systems have been aimed at overcoming previous concerns regarding toxicity and are becoming more commercially available as time goes on.16
Acknowledgment
Figure 1 through Figure 3 were provided by Elementa Oral Care.
About the Authors
Craig Callister, DMD
Private Practice, Payson, Utah
Matt Callister, DMD
Private Practice, Payson, Utah
Michael Nolan, PharmD
Clinical Pharmacist in inpatient pharmacy, US Dept of Veterans Affairs,
Ann Arbor, Michigan
Ryan Nolan, DMD
Chief Scientist, Novus Research, Provo, Utah
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Dental Caries (Tooth Decay) in Adults (Age 20 to 64). National Institute of Dental and Craniofacial Research website. https://www.nidcr.nih.gov/research/data-statistics/dental-caries/adults. Accessed January 15, 2020.
2. Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-920.
3. Burdușel AC, Gherasim O, Grumezescu AM, et al. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel). 2018;8(9). doi: 10.3390/nano8090681.
4. Angelina JTT, Ganesan S, Panicker TMR, et al. Pulsed laser deposition of silver nanoparticles on prosthetic heart valve material to prevent bacterial infection. Mater Technol. 2017;32(3):148-155.
5. Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767-2781.
6. Santoro CM, Duchsherer NL, Grainger DW. Antimicrobial efficacy and ocular cell toxicity from silver nanoparticles. Nanobiotechnology. 2007;3(2):55-65.
7. Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385-406.
8. Ibrahimkutty S, Wagener P, dos Santos Rolo, et al. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci Rep. 2015;5:16313. doi: 10.1038/srep16313.
9. Stensberg MC, Wei Q, McLamore ES, et al. Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine (Lond). 2011;6(5):879-898.
10. Yang Y, Xu S, Xu G, et al. Effects of ionic strength on physicochemical properties and toxicity of silver nanoparticles. Sci Total Environ. 2019; 647:1088-1096.
11. Torres-Méndez F, Martinez-Castañon GA, Torres-Gallegos I, et al. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent Mater J. 2017;36(3):266-274.
12. Jowkar Z, Shafiei F, Asadmanesh E, Koohpeima F. Influence of silver nanoparticles on resin-dentin bond strength durability in a self-etch and an etch-and-rinse adhesive system. Restor Dent Endod. 2019;44(2):e13.
13. Sarkar D, Khare D, Kaushal A, et al. Green and scalable synthesis of nano-silver loaded silica microparticles by spray-drying: application as antibacterial agent, catalyst and SERS substrate. Appl Nanosci. 2019;9(8):1925-1937.
14. Hembram KC, Kumar R, Kandha L, et al. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif Cells Nanomed Biotechnol. 2018;46(suppl 3):S38-S51.
15. Qing Y, Cheng L, Li R, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine. 2018;13:3311-3327.
16. Mata R, Nakkala JR, Chandra VK, et al. In vivo bio-distribution, clearance and toxicity assessment of biogenic silver and gold nanoparticles synthesized from Abutilon indicum in Wistar rats. J Trace Elem Med Biol. 2018;48:157-165.
17. Kuang X, Chen V, Xu X. Novel approaches to the control of oral microbial biofilms. Biomed Res Int. 2018;2018:6498932.
18. Vishnu Prasanna SG, Lakshmanan R. Characteristics, uses and side effects of chlorhexidine - a review. IOSR J Dent Med Sci. 2016;15(6):57-59.
19. Liu JX, Werner J, Kirsch T, et al. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J Bone Jt Infect. 2018;3(4):165-172.
20. Fromm-Dornieden C, Rembe JD, Schäfer N, et al. Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management - prospects and limitations. J Med Microbiol. 2015;64(Pt 4): 407-414.
21. Hsueh YH, Lin KS, Ke WJ, et al. The antimicrobial properties of silver nanoparticles in bacillus subtilis are mediated by released Ag+ ions. PLoS One. 2015;10(12):e0144306.
22. Al-sharani A, Al-Hajj W, Madfa A. Clinical efficacy of nanosilver and chlorhexidine in the treatment of plaque-induced gingivitis: a randomized controlled clinical trial. J Oral Res. 2018;7(7):238-244.
23. Shawky H, Basha S, El Batouti GA, Kassem AA. Evaluation of clinical and antimicrobial efficacy of silver nanoparticles and tetracycline films in the treatment of periodontal pockets. IOSR J Dent Med Sci. 2015; 14(7):113-123.
24. Wakshlak RB, Pedahzur R, Avnir D. Antibacterial activity of silver-killed bacteria: the "zombies" effect. Sci Rep. 2015;5:9555. doi: 10.1038/srep09555.
25. Qayyum S, Khan AU. Nanoparticles vs. biofilms: a battle against another paradigm of antibiotic resistance. MedChemComm. 2016;7(8):1479-1498.
26. Callister C, Callister M, Nolan M, Nolan R. Anti-caries potential of silver nanoparticles via modulation of free calcium activity within the plaque fluid of the oral biofilm: a pilot study. Dentistry. 2018;8:12. doi: 10.4172/2161-1122.1000529 .
27. Tokura T, Robinson C, Watson P, et al. Effect of pH on fluoride penetration into natural human plaque. Pediatric Dental Journal. 2012;22 (2):140-144.
28. Watson PS, Pontefract HA, Devine DA, et al. Penetration of fluoride into natural plaque biofilms. J Dent Res. 2005;84(5):451-455.
29. Scarpelli BB, Punhagui MF, Hoeppner MG, et al. In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz Dent J. 2017;28(6):738-743.
30. Freire PLL, Albuquerque AJR, Sampaio FC, et al. AgNPs: the new allies against S. Mutans biofilm - a pilot clinical trial and microbiological assay. Braz Dent J. 2017;28(4):417-422.
31. Yu OY, Mei ML, Zhao IS, et al. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent Mater. 2018;34(12):e344-e352.
32. Sanavia C, Tatullo M, Bassignani J, et al. Remineralization strategies in oral hygiene: a position paper of Italian Society of Oral Hygiene Sciences-S.I.S.I.O. Working Group. Open Dent J. 2017;11:527-538.
33. Borzabadi-Farahani A, Borzabadi E, Lynch E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol Scand. 2014;72(6):413-417.
34. Cheng L, Zhang K, Weir MD, et al. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine (Lond). 2015;10(4):627-641.
35. Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials (Basel). 2019;12(16).
36. Wang J, Li J, Guo G, et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: new insight into the antimicrobial action of silver. Sci Rep. 2016;6:32699.
37. Mousavi SM, Hashemi SA, Ghasemi Y, et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol. 2018;46(suppl 3):S855-S872.