You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Surgical extraction of impacted mandibular third molars causes trauma, with the level of necessary bone removal dependent on the tooth's position and angulation as well as the anatomy of the patient. The extraction may lead to distal root surface resorption of the second molar and residual osseous defects.1,2 Surgical treatment of impacted third molars often requires use of a full-thickness mucoperiosteal flap, bone removal by means of osteotomy to access the impacted tooth, and sectioning of the tooth to allow removal. It has been shown, however, that surgical removal of impacted mandibular third molars may result in intrabony defects (IBDs) at the distal aspect of the second molar.3-8 These complications might be greater in older patients due to reduced bone volume, slow healing, or other diseases present such as periodontitis or osteoporosis.9
Kugelberg and colleagues found that 2 years after surgery, 43.3% of cases exhibited probing pocket depths exceeding 7 mm, and 32.1% showed IBDs of more than 4 mm.10 In another study by Kugelberg, periodontal healing was compared at 2 and 4 years after impacted mandibular third molar extraction. At 2 years postoperative, 16.7% of the cases of patients aged ≤25 years had IBD of more than 4 mm compared to 40.7% in the age group >25 years, and at the 4-year re-examination the corresponding figures were 4.2% and 44.4%, respectively.5 Based on these results it may be concluded that standard surgical impacted third molar extraction could lead to a compromised periodontal status of the adjacent second molar, which might necessitate additional future surgical treatment.4
Autogenous dentin graft (ADG) prepared chairside may be used for guided bone regeneration (GBR) because it has similar bio-chemical characteristics to human bone,11,12 is osteoconductive,13-15 and pos-sesses osteoinductive properties.16-33 In 1967 Yeomans and Urist demonstrated that dentin contains bone morphogenetic proteins (BMPs), which promote the differentiation of mesenchymal stem cells into chondrocytes and, thus, enhance formation of bone.16 Additionally, both alveolar bone and teeth are derived from neural crest cells.16 Murata et al first utilized human autogenous dentin for GBR in a sinus floor lift procedure in 2003.17
A commercial dentin grinding system may be used to enable clinicians to produce a chairside ADG with a particle size ranging from 300 µm to 1200 µm that is disinfected and ready for use within 8 minutes.34 The authors evaluated the healing potential of this graft in osseous defects distal to second molars placed at the time of third molar extraction surgery, compared to a control group that underwent a standard impacted third molar extraction surgery without any additional GBR procedure.
Material and Methods
The analysis was designed as a randomized study that included both split-mouth and unilateral extraction sites. Thirteen patients were selected (three male and 10 female) with an age range of 18 to 27 years with a mean age of 22.61 years. Each patient had at least one impacted mandibular third molar tooth (IMMT), which was horizontally impacted below the cementoenamel junction of the second molar. All patients presented a healthy periodontal status prior to treatment.
The autogenous dentin was created following extraction of the IMMT utilizing the Smart Dentin Grinder™ with the protocol for mineralized dentin graft fabrication indicated. This dentin grinding system is available in the United States from KometaBio (kometabio.com), IDS-Integrated Dental Systems (idsimplants.com), and GoldenDent (physicsforceps.com).
Inclusion Criteria
To be included in the study, the patient needed to: (1) be willing to participate in the study, (2) be at least 18 years of age, (3) be in good general health and have no periodontal disease, and (4) have at least one IMMT that was horizontally inclined in relation to the second molar.
Exclusion Criteria
Patients were excluded from the study if they: (1) had active periodontal disease, (2) were underaged (<18 years old), (3) had a systemic condition that was contraindicated to undergo surgical extractions, or (4) had alcohol or drug abuse or any conditions associated with poor compliance.
Pre-experimental Treatment
After patient selection and receipt of informed consent, a full periodontal examination was performed on the patients and periodontal charting was completed. This was done to establish periodontal disease-free status and perform plaque evaluation to see if professional oral hygiene was needed.
Presurgical Procedures
Prior to IMMT surgery, each patient's medical history was recorded, including medicine usage and background diseases, and a panoramic radiograph was performed to establish the angle of impaction, the relation of the IMMT to the distal aspect of the second molar, and also whether any infectious lesions were present in the oral cavity. Study and control group patients were assigned randomly.
IMMT Surgery
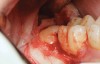
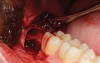
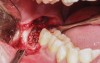
A presurgical rinse of 0.2% chlorhexidine (CHX) solution was performed, followed by administration of local anesthetic using 4% articaine with epinephrine solution. A crestal incision with a vertical releasing incision at the mesial aspect of the surgical area was made, and a full-thickness mucoperiosteal flap was elevated (Figure 1). A buccal and, if required, distal osteotomy was performed using a round carbide bur on a straight handpiece. When necessary the tooth was dissected to allow it to be elevated and extracted (Figure 2). When performing the third molar extraction care was taken to not damage the adjacent second molar tooth nor its supporting alveolar bone. The extraction socket was thoroughly debrided with hand instruments and rinsed with a 0.2% CHX solution to decrease bacteria in the socket (Figure 3).35
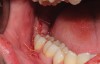
The extracted tooth fragments were cleaned to remove caries if present as well as periodontal ligament (PDL) remnants (Figure 4). The tooth was ground into particles using the dentin grinder machine. The particles were then saturated for 10 minutes in a dentin cleanser solution (sodium hydroxide solution mixed in 20% ethanol); this was followed by a phosphate buffered saline wash, resulting in a bacteria-free, autogenous graft material ready for implantation (Figure 5).
The ADG was packed into the osseous defect related to the extracted tooth and allowed to moisten with the patient's blood in the site. Sterilized gauze was used to remove any residual wetness from the site, resulting in a well-packed socket with ADG (Figure 6). The ADG was then overlaid with an absorbable hemostatic gelatin sponge, and the flap was closed by primary intent with 4-0 vicryl sutures (Figure 7). For the control group the same surgical protocol was employed except for the use of the ADG; the hemostatic sponge was placed prior to site closure. A blood clot was formed using a surgical curette and the flap was closed with 4-0 vicryl sutures.
Post-surgical Procedures
All patients received a 5-day course of prophylactic antibiotics, amoxicillin 1000 mg twice per day, and nonsteroidal anti-inflammatory drugs (NSAIDs) that were prescribed according to individual needs. The patients were instructed to rinse with 0.12% CHX-based solution twice daily for 14 days. Sutures were removed 10 days postoperatively.
Assessments and Clinical Examination
The clinical examinations and surgery were performed by an oral surgery resident in the department of Oral and Maxillofacial Surgery at Lithuanian University of Health Sciences in Kaunas, Lithuania. Probing pocket depths distal to the second molar were recorded using a manual periodontal probe from both the buccal and lingual sides.
Radiographic Examination
Panoramic radiographs were performed at 3 months and 12 months postoperatively to evaluate bone preservation/regeneration and to exclude pathologies that might have occurred from surgery. Twelve months after surgery radiographic interpretation was made using imaging software (Kodak Dental Imaging Software 6.12.32.0, Carestream Dental, carestreamdental.com) to measure alveolar bone level in the study and control groups.
Postoperative Wound Healing Potential and Its Effect on Patient
Patients were evaluated at 10 days, 3 months, and 12 months postoperatively to assess wound healing. At these appointments, patients were given a questionnaire to evaluate postoperative pain, swelling, use of NSAID, food impaction at the surgical site, and overall impact to the patient's daily life. At suture removal, patients were evaluated for whether "dry socket" (alveolar osteitis) was present or not. Patients evaluated severity from 1 to 5, with 1 referencing no discomfort/pain and 5 indicating extreme discomfort/pain. Impact of surgery on daily life also was evaluated from 1 to 5, with 1 referencing no impact on the patient's daily routine and 5 representing extreme impact on daily routine. Patients added comments to describe the extent of negative or positive impact they experienced in their daily routine. Mann-Whitney test was used to determine statistical significance utilizing SPSS Statistics version 23.0 (IBM, ibm.com). The level for significance was set at P < .05.
Ethical Requirements
All patients in this study were informed about the study design and the rights to withdraw from the study at any time according to the Declaration of Helsinki. The study was approved by the Lithuanian Health Ministry of Bioethical Committee.
Results
Table 1 and Figure 8 present the results of the mean pocket depth assessments after 3 months and 12 months in both the control and study groups. In total, 24 cases were evaluated after 3 months and 12 months (N = 11 in control group, and N = 13 in study group). Very light force was used for probing after 3 months due to incomplete healing.
At 3 months post IMMT surgery the study group demonstrated probing depths of 1.31 mm (standard deviation [SD] 0.751 mm), whereas the control group showed probing depths of 4 mm (SD 0.853 mm). At 12 months, the study group demonstrated probing depths of 1.15 mm (SD 0.801 mm), while the control group demonstrated probing depths of 4.45 mm (SD 0.934 mm).
Means among the control group after 3 months and 12 months, as well as among the study group at the same time periods, did not differ significantly. Using the Mann-Whitney test to compare differences between study and control groups, a statistically significant difference was observed between the two groups when comparing probing depths at the same time period (P < .001) (Table 2).
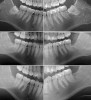
Radiographic comparison of a sample case in the study group with impacted mandibular third molars bilaterally was done following extraction and ADG placement at 3 months and 12 months post-treatment (Figure 9). The radiographs demonstrated conversion of the ADG into bone that blended with the surrounding native bone and long-term maintenance.
According to the questionnaires, five of the 11 patients in the control groupreported food impaction at the surgical site after 3 months, which required additional attention to allow for proper oral hygiene maintenance. This included patient instruction to keep the area free of food debris and maintain normal oral hygiene plus a soft diet during the initial healing phase. None of the patients from the study group reported any abnormal food impaction around the surgical site. Other than that, neither group reported any unusual events during healing.
Radiographic interpretation of alveolar bone levels after 12 months showed significantly lower alveolar bone level in the study group (P < .001) than in the control group (Table 3). Mean of alveolar bone level in the control group (N = 11) was 4.2 mm (SD 1.2 mm), and in the study group (N = 13) it was 1.05 mm (SD 0.91 mm).
Discussion
Due to the nature of patients requiring IMMT surgery who are referred to an oral surgery specialist-that is, they typically return to their referring dentist following initial healing post-surgery-postoperative follow-up is usually limited. As Kugelberg and colleagues suggested, long-term periodontal stability distal to the second mandibular molar after IMMT surgery is compromised in the absence of socket grafting at the time of extraction surgery.10
A treatment option to reduce the risk of future periodontal pathology mesial to the IMMT surgical site is the use of osseous grafting to preserve the distal aspect of the second mandibular molar. Use of commercially available osseous grafting products, however, increases the cost of treatment for the patient, which may lead to refusal for that additional procedure.
An ADG has been documented as a reliable graft source when socket preservation is being performed and for other osseous grafting applications, as it has been noted that large amounts of new woven bone formation were generated after 60 days of healing, and small amounts of lamellar bone were seen after 90 days.36 ADG has also demonstrated successful use in conjunction with implant placement, yielding osseous stability at 2 years post-treatment, and can be considered an alternative to commercially available osseous grafting products or even autogenous bone.37 Because the graft material is autogenous, it provides an abundance of BMPs attracting progenitor cells and acting as a scaffold for new bone growth. Dating back to 1967 and in subsequent articles, Urist et al identified that cells that come in contact with BMP change the pathway of differentiation and tissue development resulting in bone formation.19,38,39 Further, he identified that dentin was a source of BMP that could have the same effects on bone formation when utilized as a graft material. This was further supported in the literature, which has stated that dentin is a source of BMP that is beneficial to osseous graft development and organization when placed into tissue that has osteogenic potential.40,41 BMP extracted from human dentin matrix induced new bone formation in situ after 3 weeks when implanted. It has been reported that dentin-matrix-derived BMP is similar to, though likely not identical to, bone-matrix-derived BMP, although both types of BMP have the same action in vivo.20
Resorption of the ADG particles is slow, which, therefore, assists in lamellar bone formation with stability of the resulting bone over time. Studies have supported that cortico-cancellous bone that formed was maintained successfully with an implant after an average follow-up of 5 years.42 These results were consistent with those of other follow-up studies.
It may be noted that while the patients in the present study were of a relatively young age and could be expected to heal well, these patients were in the general age range of patients who typically have impacted molars extracted. The surgery tends to be aggressive in nature as large amounts of osteotomy are required to expose the impacted molar to allow extraction. In the authors' experience and clinical observations, when using this grafting material in older patients there have been no reports of healing ability being hampered.
Conclusion
The use of grafting at the time of surgical extraction of impacted mandibular third molars can aid in the prevention of site resorption during healing and has been documented to result in formation of osseous tissues on the distal aspect of the adjacent second molar. Various osseous graft products have been advocated, offering varying results and involving a range of costs in their utilization. As shown in this study ADG is a biologically suitable material for use in GBR following IMMT surgery. It is a cost-efficient approach for the patient and allows the surgeon to employ autologous bone grafting material, which is often preferable, for GBR. Additionally, because the resulting graft material is derived from the patient, the potential for immunological reactions that may accompany the use of commercial products is eliminated.
About the Authors
Avi Kuperschlag, DDS, MSc
Resident, Lithuanian University of Health Sciences, Kaunas, Lithuania; Private Practice specializing in Oral Surgery, Kaunas, Lithuania
Greta Keršytė
Fourth-year Dental Student, Lithuanian University of Health Sciences,
Kaunas, Lithuania
Gregori M. Kurtzman, DDS
Private Practice, Silver Spring, Maryland; Master, Academy of General Dentistry; Diplomate, International Congress of Oral Implantologists
Robert A. Horowitz, DDS
Private Practice, Scarsdale, New York
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Kugelberg CF, Ahlström U, Ericson S, et al. The influence of anatomical, pathophysiological and other factors on periodontal healing after impacted lower third molar surgery. A multiple regression analysis. J Clin Periodontol. 1991;18(1):37-43.
2. Dodson TB. Reconstruction of alveolar bone defects after extraction of mandibular third molars: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(3):241-247.
3. Coceancig PL. Alveolar bone grafts distal to the lower second molar. J Maxillofac Oral Surg. 2009;8(1):22-26.
4. Kugelberg CF. Impacted lower third molars and periodontal health. An epidemiological, methodological, retrospective and prospective clinical, study. Swed Dent J Suppl. 1990;68:1-52.
5. Kugelberg CF. Periodontal healing two and four years after impacted lower third molar surgery. A comparative retrospective study. Int J Oral Maxillofac Surg. 1990;19(6):341-345.
6. Sammartino G, Tia M, Marenzi G, et al. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63(6):766-770.
7. Efeoglu C, Akçay YD, Ertürk S. A modified method for preparing platelet-rich plasma: an experimental study. J Oral Maxillofac Surg. 2004;62(11):1403-1407.
8. Kaul RP, Godhi SS, Singh A. Autologous platelet rich plasma after third molar surgery: a comparative study. J Maxillofac Oral Surg. 2012;11(2):200-205.
9. Marmary Y, Brayer L, Tzukert A, Feller L. Alveolar bone repair following extraction of impacted mandibular third molars. Oral Surg Oral Med Oral Pathol. 1986;61(4):324-326.
10. Kugelberg CF, Ahlström U, Ericson S, Hugoson A. Periodontal healing after impacted lower third molar surgery. A retrospective study. Int J Oral Surg. 1985;14(1):29-40.
11. Finkelman RD, Mohan S, Jennings JC, et al. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990;5(7):717-723.
12. Huggins C, Wiseman S, Reddi AH. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med. 1970;132(6):1250-1258.
13. Kim YK, Lee JK, Kim KW, et al. Healing mechanism and clinical application of autogenous tooth bone graft material. In: Pignatello R, ed. Advances in Biomaterials Science and Biomedical Applications. London: IntechOpen; 2013. doi: 10.5772/53200.
14. Kim YK, Kim SG, Yun PY. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):e39-e45.
15. Al-Namnam NM, Shanmuhasuntharam P, Ha KO, Siar CH. Processed allogenic dentine as a scaffold for bone healing: an in vivo study. Australian Journal of Basic and Applied Sciences. 2010;4(12):5932-5940.
16. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12(8):999-1008.
17. Murata M, Akazawa T, Mitsugi M, et al. Autograft of dentin materials for bone regeneration. In: Pignatello R, ed. Advances in Biomaterials Science and Biomedical Applications. London: IntechOpen; 2013. doi: 10.5772/53665.
18. Morotome Y, Goseki-Sone M, Ishikawa I, Oida S. Gene expression of growth and differentiation factors-5, -6, and -7 in developing bovine tooth at the root forming stage. Biochem Biophys Res Commun. 1998;244(1):85-90.
19. Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50
(6):1392-1406.
20. Bessho K, Tanaka N, Matsumoto J, et al. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991;70(3):171-175.
21. Inoue T, Deporter DA, Melcher AH. Induction of cartilage and bone by dentin demineralized in citric acid. J Periodontal Res. 1986;21(3):243-255.
22. Kawai T, Urist MR. Bovine tooth-derived bone morphogenetic protein. J Dent Res. 1989;68(6):1069-1074.
23. Feng JQ, Luan X, Wallace J, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273(16):9457-9464.
24. Ritchie HH, Ritchie DG, Wang LH. Six decades of dentinogenesis research. Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci. 1998;106(suppl 1):211-220.
25. Kim YK, Choi YH. Tooth autotransplantation with autogenous tooth-bone graft: a case report. J Kor Dent Sci. 2011;4(2):79-84.
26. Moharamzadeh K, Freeman C, Blackwood K. Processed bovine dentine as a bone substitute. Br J Oral Maxillofac Surg. 2008;46(2):110-113.
27. Kim YK, Kim SG, Byeon JH, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):496-503.
28. Park SM, Um IW, Kim YK, Kim KW. Clinical application of auto-tooth bone graft material. J Korean Assoc Oral Maxillofac Surg. 2012;38(1):2-8.
29. Lee JY, Kim YK. Retrospective cohort study of autogenous tooth bone graft. Oral Biol Res. 2012;36:39-43.
30. Jeong KI, Kim SG, Kim YK, et al. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20(6):471-475.
31. Lee JY, Kim YK, Kim SG, Lim SC. Histomorphometric study of sinus bone graft using various graft material. J Dent Rehabil Appl Sci. 2011;27:141-147.
32. Kim SG, Kim YK, Lim SC, et al. Histomorphometric analysis of bone graft using autogenous tooth bone graft. Implantology. 2011;15:134-141.
33. Lee JY, Lee J, Kim YK. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J Dent Sci. 2013;8(3):281-286.
34. KometaBio, The Smart Dentin Grinder™ Autologous Dentin Grafting Protocol. Fort Lee, NJ: KometaBio; 2015.
35. Sawada K, Fujioka-Kobayashi M, Kobayashi E, et al. Effects of antiseptic solutions commonly used in dentistry on bone viability, bone morphology, and release of growth factors. J Oral Maxillofac Surg. 2016;74(2):247-254.
36. Calvo-Guirado JL, Cegarra Del Pino P, Sapoznikov L, et al. A new procedure for processing extracted teeth for immediate grafting in post-extraction sockets. An experimental study in American Fox Hound dogs. Ann Anat. 2018;217:14-23.
37. Minamizato T, Koga T, I T, et al. Clinical application of autogenous partially demineralized dentin matrix prepared immediately after extraction for alveolar bone regeneration in implant dentistry: a pilot study. Int J Oral Maxillofac Surg. 2018;47(1):125-132.
38. Urist MR, Silverman BF, Büring K, et al. The bone induction principle. Clin Orthop Relat Res. 1967;53:243-283.
39. Butler WT, Mikulski A, Urist MR, et al. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977;56(3):228-232.
40. de Oliveira GS, Miziara MN, Silva ER, et al. Enhanced bone formation during healing process of tooth sockets filled with demineralized human dentine matrix. Aust Dent J. 2013;58(3):326-332.
41. Al-Asfour A, Farzad P, Andersson L, et al. Host tissue reactions of non-demineralized autogenic and xenogenic dentin blocks implanted in a non-osteogenic environment. An experimental study in rabbits. Dent Traumatol. 2014;30(3):198-203.
42. Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: long-term follow-up. J Oral Maxillofac Surg. 2016;74(3):515.e1-e9.