You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Loss of teeth in the posterior region of the maxilla is frequently accompanied by loss of available bone volume for implant placement due to both atrophy of the residual crestal bone and enlargement of the maxillary sinus. The longer the teeth have been missing, the greater the potential loss of osseous structure to accommodate implants. This necessitates augmentation of the maxillary sinus to provide adequate bone for implant placement. Clinically, this can be performed simultaneously with implant placement when the crestal bone has sufficient height to facilitate stability of the implant and permit a crestal approach. Typically, if the height increase needed for implant placement is 5 mm or less, a crestal approach may be utilized.1 Should more height be needed or if there is insufficient height to allow for stability of the implants at the time of sinus augmentation, a lateral sinus approach may offer a better method. When a tooth is to be extracted in preparation for implant placement and a sinus lift is anticipated, if the socket will not permit stability of the implant at that time, socket grafting should be considered to better prepare the site for implant procedures.
Maxillary sinus augmentation with various bone graft materials has been a routine treatment for almost 5 decades. Transalveolar sinus floor elevation (ie, subantral augmentation) was first described by Boyne, and Tatum was credited as the innovator of the technique.2 Later, Tatum published material on sinus augmentation, and the technique became routine for the management of deficient posterior maxillary bone when implants were to be utilized.3 Subsequently, Summers proposed the use of a crestal approach with osteotomes when sufficient height was present.4
Numerous studies have reported highly successful implant survival rates when they are placed into an augmented sinus.5,6,7 Various materials have been utilized, including allografts, xenografts, and synthetics, in combination with autogenous bone graft or alone, with varying levels of success as measured by the amount of graft maturation, its density, and its ability to support implants following healing.
Autologous Blood Concentrates
As the use of autologous blood concentrates has increased in dental surgery for both soft- and hard-tissue applications, so has their use in sinus augmentation procedures. Platelet-rich plasma (PRP) was the first blood-derived product utilized in dentistry, and it was subsequently combined with osseous materials to create grafts for sinus augmentation.8 However, meta-analysis has suggested that PRP combined with osseous graft material has no influence on bone formation and implant survival in maxillary sinus augmentation.9
The next generation of platelet concentrates, platelet-rich fibrin (PRF), allowed for simplified processing without biochemical blood handling. Sinus floor augmentation using PRF combined with freeze-dried bone allograft has been reported to result in a reduction in healing time prior to implant placement.10Another study demonstrated that when PRF was added to the bone graft material, histology results collected after 106 days revealed lamellar bone tissue with an interposed stroma that appeared relaxed and richly vascularized.11 The use of PRF as the sole sinus augmentation material (ie, not in combination with any osseous graft material) has also been explored. One study concluded that, due to the presence of stem cells in PRF, sinus elevation using PRF alone may promote natural bone regeneration to support implants either placed simultaneously or in a delayed approach.12
Evolution of PRF
The development of PRF was first described by Choukroun in 2001.13 He identified it as a simple, natural, and inexpensive technique for the production of leukocyte and PRF (L-PRF) concentrates using patient blood collected without anticoagulant and immediately centrifuged (2,700 rpm/12 minutes).14,15 PRF is a fibrin biomaterial comparable to PRP-not an improved fibrin glue.16PRP has poor physical properties and takes a long time for centrifugation. Its fast release exists only in liquid form and contains anticoagulant, which stops platelets from the natural coagulation cascade and hampers the growth factors that will be needed for regeneration.
During the centrifugation of PRF, coagulation begins, and three areas quickly differentiate in the tube: a red blood cell base at the bottom, a platelet-poor acellular plasma as a supernatant at the top, and the PRF clot in between these two layers. PRF concentrate consists of mostly platelets and more than half of the leukocytes present in the collected blood.17,18 The platelets merged within the fibrin clot mesh together like a cement, but the leukocytes within this dense 3-dimensional fibrin network are alive and functional.19 PRF releases growth factors, including vascular endothelial growth factor (VEGF), transforming growth factor beta 1 (TGF-b1), platelet-derived growth factor AB (PDGF-AB), and matrix glycoproteins (eg, thrombospondin-1), in large amounts during the first 7 days at the localized site.20
Advanced platelet-rich fibrin (A-PRF) (1,500 rpm/14 minutes) was introduced in 2013. Chouckroun and Ghanatti modified their original blood centrifuging process to incorporate a low-speed centrifugation concept.21 The concept, published in 2017, further reduced the centrifugation force and time (1,300 rpm/8 minutes), resulting in an increase in the number of white blood cells and platelets. This biomaterial, which they termed advanced platelet-rich fibrin plus (A-PRF+), could also be transformed into a membrane or a plug. In addition, an injectable form of platelet-rich fibrin (i-PRF) can be collected from the top 1-ml layer of centrifugation tubes after blood is processed at 700 rpm for 3 minutes. These new biologics contain a higher concentration of monocytes responsible for the release of bone morphogenetic proteins (BMPs) and are a great source of BMP-2 and BMP-7. This positions A-PRF+ as a superior, multipotent blood enhancement product when compared with the use of standard PRF or PRP in surgical procedures.
Applications for PRF have been described in the literature regarding oral maxillofacial surgery, implant surgery, periodontal regenerative surgery, and facial esthetics.22 Clinically, PRF stimulates numerous types of cells, but particularly influences the proliferation and differentiation of osteoblasts. For a number of years, research has advocated for the use of PRF during lateral sinus lift procedures and crestal osteotome augmentation, reporting improvement in clinical outcomes when compared with procedures performed without PRF.23,24,25 In studies involving sinus augmentation utilizing PRF in combination with osseous bone materials, histological evaluations revealed that new bone formation had occured.23,24,25 In addition, the use of this mixed graft material reduced healing time. Another study demonstrated that the formation of new bone increased when sinus augmentation was performed with bovine bone mixed with PRF.26 Although PRF is effective in the early phases of wound healing, its effectiveness may change depending on the characteristics of the accompanying graft materials.27 However, it remains superior to the use of PRP, which when added to bone graft materials in sinus augmentation and evaluated histologically, demonstrates no measurable beneficial effect on wound healing or bone remodeling.28
The use of granular osseous materials is associated with increased costs, risk of infection, and the potential for adverse patient reactions; therefore, using PRF as the sole graft material can be useful because it is easily obtained, cost-effective, and promotes natural bone regeneration.29 A systematic review and analysis found that the addition of osseous graft materials is not necessary to achieve height for simultaneous implant placement when the crest's height at the time of surgery is adequate enough to ensure implant stability.30 The use of PRF as the sole sinus augmentation material during simultaneous sinus lift and implant placement is a reliable surgical option that promotes natural bone regeneration without the need for osseous graft materials.31 It is also recommended for patients with narrow maxillary sinus anatomy.
Case Report
A 49-year-old female patient presented with pain in her right maxillary first molar, which had previously been endodontically treated. The patient's medical history indicated the presence of hypertension and thyroid disease, both of which were being managed by medication. Clinical and radiographic examinations determined that the tooth was not re-treatable endodontically. The patient was informed of the findings, and treatment options were discussed, which, following extraction of the tooth, included placement of a prosthesis fixed to the adjacent natural teeth or socket grafting followed by a single tooth implant after site healing. The patient did not wish to have the adjacent teeth prepared, so she selected the implant option.
Extraction and Socket Grafting
When the patient returned for treatment, anesthesia was administered in local infiltrations around the molar that would be extracted. The affected molar was atraumatically extracted and the socket (ie, type 1 Salama classification) was thoroughly curetted to remove any granulation tissue and debris related to the failed endodontic treatment.
The bone graft was prepared in a sterile dish using two osseous graft materials that were selected for their individual properties. The first was a next-generation synthetic hydroxyapatite with high porosity and surface area. It provides long-term stability and compression resistance with a slow resorption profile. This material is useful for repair procedures in which new bone development may be difficult to achieve, such as those involving failed endodontic extraction sockets. The second material, a natural bone mineral matrix, was an equine-derived bone graft substitute with a complex, porous network that closely resembles natural human bone. The result of this combination is a natural bone mineral matrix with osteoconductive properties. The properties of both products compliment each other, helping to achieve a better fill of the extraction socket, either for later implant placement or to preserve the ridge's anatomy and prevent the resorption normally encountered with sockets that are left unfilled following extraction.
The prepared extraction socket was filled with the graft mixture and covered with a resorbable collagen membrane. A figure eight suture using a 6-0 polypropylene blue monofilament was placed to retain the membrane and socket graft during the initial days of healing. When the patient returned a week later and the suture was removed, the site demonstrated minimal inflammation and partial closure of the gingiva was observed.
Surgical Planning
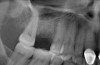
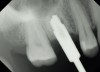
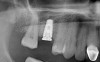
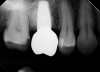
Due to the need for medical treatment for shoulder and neck issues, the patient was delayed in returning to the office for subsequent evaluation. At the 7-month postoperative appointment, the socket was assessed and a panoramic radiograph was taken to evaluate the bone at the planned implant site (Figure 1). The socket was filled with bone that was indistinguishable from the patient's native bone adjacent to the site. Insufficient height to place an implant was noted, which would require a crestal sinus lift prior to implant placement. A cone-beam computed tomography (CBCT) scan was ordered for planning purposes. Planning software was used to properly position a 5.2 x 10 mm implant for placement into the edentulous site, confirming that some elevation of the sinus would be required (Figure 2 and Figure 3). This data was used to order a surgical stent to guide implant placement (Figure 4).
Sinus Lift and Implant Placement with A-PRF+
At the following appointment, anesthesia was administered as a local infiltration, and three tubes of blood were drawn from the patient. They were placed into the centrifuge and spun at 1,300 rpm for 8 minutes to create A-PRF+ plugs. Following centrifuging, the A-PRF+ plugs were removed from the tubes, separated from the red cells with a scissor, and placed into the PRF box. The PRF box uses light compression to remove excess plasma liquid from the plugs, readying them for intraoral use.
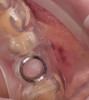
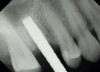
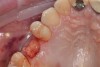
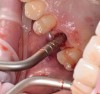
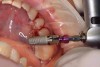
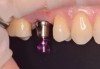
After the surgical stent was tried in and verified (Figure 5), a 5-mm tissue punch was introduced through the surgical stent to remove a core of soft tissue where the implant would be placed. Osteotomy drills matching the implant system to be used were introduced through the surgical stent and taken to the final diameter. The stent limited the depth to 2-mm short of the sinus floor. The stent was removed, the final drill was reinserted into the osteotomy, and a periapical radiograph was taken to verify the depth and orientation to the sinus floor (Figure 6). Next, a 5-mm wide, cupped-end sinus osteotome was introduced into the osteotomy, and another periapical radiograph was taken to check the depth of the osteotomy in relation to the sinus floor. Using a surgical mallet, the osteotome was advanced an additional 4 mm with gentle tapping to elevate the sinus floor atraumatically (Figure 7). A blunt instrument was used to verify the integrity of the sinus membrane and confirm that perforation had not occurred during the sinus lift. Following verification of sinus integrity, the previously created A-PRF+ plugs (Figure 8) were removed from the PRF box and individually placed into the osteotomy (Figure 9). The 5-mm osteotome was used to efficiently press the plug into the osteotomy, filling the space created between the sinus floor and the sinus membrane (Figure 10). The 5.2 x 10 mm implant with fixture mount attached was picked up with the handpiece driver on a surgical handpiece (Figure 11). The implant was inserted with the handpiece using 20 rpms until an insertion torque of 40 Ncm was reached. Final seating of the implant was accomplished with a torque wrench, which was used until the implant was positioned at the correct depth in relation to the site's crestal bone and the cementoenamel junction of the adjacent teeth (Figure 12). The fixture mount was removed, a cover screw was placed into the implant, and a periapical radiograph was taken to verify placement (Figure 13). Because the A-PRF+ is radiolucent when initially placed, its position cannot be ascertained radiographically at the time of surgery. The patient was scheduled for a 1-week postoperative check, at which time the cover screw was visible with slight irritation of the gingiva around its periphery. At the 3-week postoperative examination, the cover screw remained exposed, but the peripheral gingival inflammation was absent and the tissue appeared normal in color and tone.
Restoration
The patient returned 6 months later to begin the restorative phase of treatment. By this time, the gingiva had partially covered the cover screw. After local anesthesia was achieved, a 5-mm tissue punch was used to fully expose the cover screw, and it was removed. The fixture mount that came with the implant system was designed to be used as a closed-tray impression coping and as a stock abutment after the colored top portion of the mount has been separated. A periapical radiograph was taken to verify full seating of the abutment into the implant. A light body vinyl polysiloxane (VPS) impression material was syringed around the fixture mount intraorally, and a stock tray filled with medium body VPS material was inserted into the mouth for a closed-tray impression and allowed to set. Upon setting, the tray was removed and a healing abutment was inserted into the implant. An impression of the opposing arch was taken with medium body VPS material, a bite was taken with bite registration material, and both were sent to the lab.
To fabricate a soft-tissue model, the lab placed an analog onto the fixture mount and reinserted it into the impression. A scan head was then inserted into the analog in the soft-tissue model to create a virtual model. In the planning software, a titanium base was placed into the virtual analog and a crown was designed to fit the base and the anatomical parameters of the available space. Following design, the full-contour zirconia crown was milled and polished. The finished monolithic zirconia crown was then luted to a titanium base insert with a self-adhesive resin cement, and upon seating, the excess cement was removed and the margin between the base and the zirconia was polished.
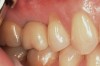
After the restoration was returned from the lab and steam sterilized, the healing abutment was removed, the crown was tried in, and a periapical radiograph was taken to verify seating. With the seating verified, the crown's fixation screw was tightened with a torque wrench, and the access hole was sealed with composite. A final check revealed the presence of a functional occlusion.
Conclusion
After 36 months, the patient was recalled to the office and a radiograph was taken to evaluate the sinus augmentation and crestal bone level following extended function (Figure 14). The crestal bone had remained stable, and no evidence of resorption was noted. The density of the sinus graft was similar to that of the native bone that was present inferior to the augmented sinus area, showing the successful conversion of the A-PRF+ and its natural components into bone without the need for osseous graft materials.30 Regarding the soft-tissue response, the gingiva had remained in a stable position and demonstrated no evidence of inflammation (Figure 15). The patient expressed her satisfaction with the final result.
About the Authors
Delia Tuttle, DDS
Private Practice
Lake Elsinore, California
Gregori M. Kurtzman, DDS
Private Practice
Silver Spring, Maryland
References
1. Baumann A, Ewers R. [Minimally invasive sinus lift. Limits and possibilities in the atrophic maxilla]. Mund Kiefer Gesichtschir. 1999;3(Suppl 1):S70-S73.
2. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38(8):613-616.
3. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30(2):207-229.
4. Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994;15(2):152,154-6,158 passim;quiz 162.
5. Blomqvist JE, Alberius P, Isaksson S. Two maxillary sinus reconstruction with endosseous implants: A prospective study. Int J Oral Maxillofac Implants. 1998;13(6):758-766.
6. Valentini P, Abensur DJ. Maxillary sinus grafting with anorganic bovine bone: A clinical report of long-term results. Int J Oral Maxillofac Implants. 2003;18(4):556-560.
7. Tong DC, Rioux K, Drangsholt M, et al. A review of survival rates for implants placed in grafted maxillary sinuses using meta-analysis. Int J Oral Maxillofac Implants. 1998;13(2):175-182.
8. Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71(10):1654-1661.
9. Lemos CA, Mello CC, dos Santos DM, et al. Effects of platelet-rich plasma in association with bone grafts in maxillary sinus augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45(4):517-525.
10. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
11. Tatullo M, Marrelli M, Cassetta M, et al. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9(10):872-880.
12. Tajima N, Ohba S, Sawase T, et al. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants. 2013;28(1):77-83.
13. Choukroun J, Adda F, Schoeffler C, et al. PRF: An opportunity in perio-implantology. Implantodontie. 2000;42:55-62.
14. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167.
15. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-e44.
16. Dohan DM, Choukroun J. PRP, cPRP, PRF, PRG, PRGF, FC … How to find your way in the jungle of platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):305-306.
17. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-e50.
18. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-e55.
19. Dohan Ehrenfest DM, Diss A, Odin G, et al. In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):341-352.
20. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, et al. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63-69.
21. Choukroun J, Ghanaati S. Introducing the low-speed centrifugation concept. In: Miron RJ, Choukroun J eds. Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications: Biological Background and Clinical Indications, One. Hoboken, NJ: John Wiley & Sons;2017:33-46.
22. Mazor Z, Horowitz RA, Del Corso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol. 2009;80(12):2056-2064.
23. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
24. Simonpieri A, Del Corso M, Sammartino G, et al. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: a new grafting protocol. Implant Dent. 2009;18(2):102-111.
25. Simonpieri A, Del Corso M, Sammartino G, et al. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part II: implant surgery, prosthodontics and survival. Implant Dent. 2009;18(3):220-229.
26. Tanaka H, Toyoshima T, Atsuta I, et al. Additional effects of platelet-rich fibrin on bone regeneration in sinus augmentation with deproteinized bovine bone mineral: preliminary results. Implant Dent. 2015;24(6):669-674.
27. Bolukbasi N, Ersanlı S, Keklikoglu N, et al. Sinus augmentation with platelet rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol. 2015;41(5):586-595.
28. Raghoebar GM, Schortinghuis J, Liem et al. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16(3):349-356.
29. Aoki N, Kanayama T, Maeda M, et al. Sinus augmentation by platelet-rich fibrin alone: a report of two cases with histological examinations. Case Rep Dent. 2016;2016:2654645. Epub 2016 Oct 3.
30. Pérez-Martínez S, Martorell-Calatayud L, Peñarrocha-Oltra D, et al. Indirect sinus lift without bone graft material: Systematic review and meta-analysis. J Clin Exp Dent. 2015;7(2):e316-e319. doi: 10.4317/jced.51716.
31. Simonpieri A, Choukroun J, Del Corso M, et al. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent. 2011;20(1):2-12.