You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The late Dr. Morton Amsterdam astutely wrote, "There may be different ways of treating a disease, but there can only be but one correct diagnosis."1 The changing culture and landscape of interdisciplinary dentofacial therapy (IDT) suggests the need for a symbiosis of the primary care provider and specialist for the effective management of dental, periodontal, and craniofacial issues. The goal of such collaborative work is to accurately and comprehensively diagnose and prognosticate craniofacial deficiencies. The unique perspectives and knowledge of each medico-dental professional is incorporated into an ideal plan for each individual patient. This collaborative IDT requires the leadership of the patient's restorative dentist to coordinate and amalgamate input from each specialist involved.
Evolutionary changes over the past three centuries have contributed to the current prevalence of the phenomenon of "facial recession."2 This progressively retrognathic maxillary and mandibular positioning is the result of the evolutionary and cultural demands to develop a more pronounced frontal lobe of the brain. The endpoint of IDT is to re-establish the homeostatic balance between the craniofacial structures and the periodontium that they support. This is necessary to obtain oral health that can be sustained over a lifetime but cannot be achieved without a thorough understanding of the embryologic and developmental processes leading to craniofacial anatomy.
Contemporary IDT begins with a facially prioritized approach and often requires the interdisciplinary team to re-establish tooth position and proportions. Space appropriation of the teeth is critical to the dentofacial management of the stomatognathic system and often requires optimizing root position within a sound periodontal foundation. This becomes especially crucial when wear (through attrition and erosion) and compensatory tooth movement (dental compensations) have occurred.
Dental compensations occur as a result of a skeletal disharmony and are commonly seen whenever anterior-posterior or transverse maxilla-mandibular disharmonies are present.3,4 Dental crowding is often an arch length deficiency, which corresponds to a deficiency in dentoalveolar bone volume. This limits the opportunities for dental expansion and often necessitates extractions to reconcile the existing tooth mass to the available arch length. Imaging-based software programs such as Digital Smile Design (DSD) (digitalsmiledesign.com), Suresmile® (suresmile.com), or Nemotec (nemotec.com) can help bridge the gap between a facially prioritized treatment plan and optimal, patient-centered results. Such software provides greater clarity, precision, and accuracy when transitioning from patient expectations to realized endpoints.
The 3-dimensional (3D) cone-beam computed tomography (CBCT) imaging component of these planning software programs represents a paradigm shift in IDT. Incorporating 3D regional anatomy into virtual planning allows a biologic conscience to guide the clinician during dental and orthodontic planning.5 If the facially prioritized treatment plan calls for the tooth position outside of the dentoalveolar bone volume limits, alternative approaches to orthodontic tooth movement must be considered. Treatment such as surgically facilitated orthodontic therapy (SFOT) may allow the IDT team to accomplish outcome goals and avoid iatrogenic complications. Collaborative treatment planning by a cohesive IDT team must occur prior to embarking on a restorative rehabilitation or orthodontic treatment that may exceed the boundaries of the "orthodontic walls."6 Failure to respect these boundaries may lead to unstable and potentially harmful results.
The ever-important but often deficient (especially in the anterior sextant) facial bone thickness must be considered during treatment planning.7,8 The use of CBCT is critical in assessing dentoalveolar, alveoloskeletal, and skeletal relationships (as well as the anatomic structures of the temporomandibular joint) during the comprehensive treatment planning process.5
The aim of this article is to highlight the importance of contemporary IDT and expatiate on the benefits of expanded orthodontic approaches such as SFOT in the context of managing the natural dentition.
Mouth, Bone, and Airway Volumes: Historical and Evolutionary Perspectives
The prevalence of an ideal dentofacial condition currently may be decreasing among some populations.9 Conversely, tooth crowding, retrognathia, deficient dentoalveolar bone, and other dentofacial abnormalities in both maxillary and mandibular osseous structures are widespread.10,11 These anomalies may be quite prevalent, but they cannot necessarily be considered a variation of normal. Although these maladies have always existed, new tools are now available in the dental armamentarium to deal with them. Mandelaris et al have described case type patterns of common malocclusions that can be useful in identifying situations where SFOT can be helpful in IDT.5
The exact reason for the increase in incidences of malocclusion patterns is not known for certain. However, their rise has been correlated with the increased consumption of highly processed foods12 and decreased breastfeeding.13-15 The imposition of a uniformly soft diet upon infants and toddlers, as well as the resulting failure to develop forward tongue and lip muscular habits, may reduce oral cavity volume (OCV). Subsequently, the impaired development of craniofacial-respiratory structures may manifest as deficient dentoalveolar bone16,17 as well as impaired development of the maxilla and mandible (ie, hypoplasia and/or retrognathia). There has been an increasing incidence of these issues for approximately 250 years.18,19
The position of teeth within the mouth represents a homeostatic relationship between the opposing forces of the lips, tongue, oral musculature, and alveolar bone. These tissue structures define a certain OCV. The teeth develop independently of the soft-tissue structures and require a specific volume in order to properly align within the dental arch. When the volume within the oral cavity is deficient compared to the volume required by the teeth, significant malocclusions can occur. In these cases, intraoral forces may move teeth into positions to compensate (dental compensations) for the skeletal imbalance, and dental crowding may occur.20 Crowding of the natural dentition is frequently accompanied by suboptimal alveolar bone thickness, resulting in dehiscences and fenestrations,21 which then limit the extent to which teeth can be safely decompensated (ie, orthodontically moved).
The combined impact of these abnormalities on oropharyngeal airway volume is an emerging focus of contemporary IDT. The most common manifestation of a suboptimal airway space is obstructive sleep apnea (OSA), which has reached epidemic proportions in both adults22,23 and children.24,25 This has resulted in controversy, as some research demonstrates that a reduced OCV (ie, increased tongue volume:OCV ratio) may trend patients toward sleep-disordered breathing conditions,26 yet other publications suggest that the extraction of four bicuspids does not influence sleep apnea conditions or esthetic perceptions of changes in facial profile.27,28
Orthodontic Decompensation: Re-establishing Homeostasis by Re-envisioning Outcomes
As a member of the IDT team, the orthodontist has the critical job of engineering tooth movement and architecting a facially prioritized plan. He or she is responsible for correcting the dental compensations that nature facilitated in response to space misappropriation. This process, known as decompensation, includes alignment of the arches in preparation for orthognathic surgery (OGS) when a true alveoloskeletal or skeletal discrepancy exists.
The primary role of SFOT is to enhance decompensations and ensure that the dentition remains within alveolar bone. This occurs through corticotomies, dentoalveolar decortication, and bone augmentation surgery. The "bone injury" created through this procedure results in a transient demineralization of the alveolar bone matrix. Tooth movement is more expeditious through the matrix while this demineralization process is occurring-a finding known as the regional acceleratory phenomenon (RAP).Because this effect is transient, there must be close collaboration among the interdisciplinary team to maximize outcome benefits of advanced dentoalveolar bone surgery. Other approaches that involve devices to provide nonsurgical methods of accelerated orthodontic tooth movement (eg, vibration or low-level light) have reasonable evidence showing that they are capable of significantly decreasing orthodontic treatment time via inducing RAP. However, without the physical augmentation of the dentoalveolar bone phenotype and available bone into which teeth can be moved (via bone grafting), the extent to which the orthodontic boundary conditions can be increased may be limited and may not allow for key treatment plan objectives to be accomplished (such as avoiding extraction/retraction movement and ensuring dentoalveolar bone anatomy/orthodontic boundary conditions are respected following tooth movement).5
The goals/rationale for SFOT are delineated in Table 1. Key phases and features of RAP are summarized in Table 2 with an emphasis on its applicability to SFOT,29-34 while Table 3 provides a historical perspective.35,36
SFOT allows the surgeon to augment the periodontium with dentoalveolar deficiencies in order to expand the scope of decompensation within the arch (interarch dental alignment) and between the arches (intra-arch relationship). This increases the volume of dentoalveolar bone and reduces the potential for incidence of orthodontic relapse.29 A detailed and thorough systematic review on corticotomy-assisted orthodontia was recently published by Zimmo et al.37
While SFOT is an advanced periodontal regeneration and/or dentoalveolar bone augmentation surgery, its primary role is not to correct significant skeletal discrepancies or dentofacial disharmonies or expedite tooth movement. It can, however, improve periodontal and dentoalveolar bone phenotype conditions for orthodontic tooth movement or future OGS. It also can improve "orthodontic camouflage" of mild skeletal conditions by expanding tooth movement possibilities when OGS is not an acceptable treatment. By enhancing the dentoaveolar bone volume, SFOT creates a biologic environment that leads to more stable orthodontic results and, in some instances, less extensive OGS, thus supporting the facially prioritized treatment plan.
When planning IDT using a facially prioritized approach, relationships at three levels can be considered during comprehensive treatment planning: (1) teeth-to-teeth relationships, (2) teeth-to-jaws relationships, (3) jaw-to-jaw relationships, and (4) jaws-to-face relationships. Optimizing teeth-to-teeth relationships may occur within the orthodontic boundary conditions and via contemporary means. Optimizing teeth-to-jaws relationships often presents the orthodontist with limitations of dentoalveolar bone volume. In order to optimize tooth movement and produce outcomes that maintain teeth within sound orthodontic boundary conditions, dentoalveolar bone augmentation via SFOT may be needed.5,29 Recently, the American Academy of Periodontology's Best Evidence Consensus on CBCT and IDT was published.5 A systematic review focusing on the dentoalveolar bone changes influenced by tooth movement highlights case type patterns of malocclusion and the benefits of SFOT to augment the phenotype during the management of "teeth to jaws" decompensation.5 Further, this review describes how in some cases SFOT can simplify and optimize conditions for more predictable OGS in patients with severe dentofacial disharmonies (jaws-to-face relationship and management).
Re-evaluating the Goals of IDT Planning
Contemporary, comprehensive IDT-based collaboration involves at least the restorative dentist and/or prosthodontist, periodontist, orthodontist, oral and maxillofacial surgeon, and endodontist. In some cases, an otolaryngologist/ear-nose-throat (ENT) specialist, sleep physician, and myofunctional therapist also may be needed in order to make correct diagnoses, establish prognoses, and develop a personalized treatment plan.
The SFOT IDT workflow is as follows: It begins with the new-patient interview, followed by patient examination and gathering of comprehensive records (in one or two visits). Conducted by either the general dentist or prosthodontist (whoever is responsible for restorative oversight and leadership), this includes obtaining clinical data through comprehensive exam, photographs, high-resolution pulse oximetry (HRPO)/home sleep study, intraoral scanning, and mounted study models, and imaging diagnostics via full-mouth digital radiographs, CBCT imaging, and magnetic resonance imaging (MRI) when needed. The next step in the workflow is co-discovery and comprehensive treatment planning, with input from team members representing periodontics, orthodontics, oral and maxillofacial surgery, and any others needed, such as primary care physician, ENT, pediatrics, endodontics, and the dental laboratory. In grand rounds, all the IDT team members evaluate the gathered patient data, and an action plan, steered by restorative goals, is generated. The following steps are then: (1) disease control and provisionalization (pre-SFOT restorative therapy), (2) pre-SFOT orthodontics, (3) SFOT surgery, (4) SFOT orthodontics, (5) interim orthodontic transitional restorations, (6) orthodontic refinement and finishing, (7) orthognathic surgery (if required), (8) prosthetic phase completion as determined by esthetic and functional goals, and, finally, (9) supportive periodontal maintenance.
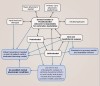
The treatment synthesis of the SFOT workflow used in contemporary IDT is exhibited in Figure 1.
IDT has to consider the aforementioned limitations of the existing dentoalveolar bone, the volume of which will dictate the extent to which tooth movement is biologically safe. This determinant of tooth movement has been termed the "orthodontic walls"6 and is often a limiting factor in treatment due to inadequate bone thickness.38 In 2013, Mandelaris et al published a CBCT-based classification system that can be utilized prior to orthodontic therapy to help determine the relative safety and risk of tooth movement.39 This system evaluates the thickness of both the crestal and radicular dentoalveolar bone (thick, >1 mm; or thin, <1 mm) for the classification of patients into four distinct phenotypical presentations. The use of such a system may help not only in determining but also in conveying the importance of alternative orthodontic approaches, such as SFOT.
Restorative Leadership
Frequently, aberrant tooth position and structural compromise of the dentition (from attrition and erosion) make ideal dentistry impossible. In such cases, orthodontic treatment becomes necessary to re-appropriate space in order to facilitate anatomically correct prosthetic dentistry. However, the benefits of idealizing tooth form and position are not limited to restorative outcomes. In the contemporary IDT paradigm, the recognition of airway problems (frequently accompanied by retrognathia40,41) as a component of dental-related treatment planning is critically important. This emerging scope of the restorative dental practice should emphasize early and, of course, accurate diagnosis and prevention. From a dental perspective, the outcome goal of airway management can be envisioned in terms of expanding OCV in conjunction with hard-tissue space appropriation to allow for ideal functional and esthetic prosthodontics.
Sleep-disordered breathing conditions are not unlike other systemic health problems in that if they are diagnosed and treated early, fewer negative sequelae and more favorable outcomes will likely result. Even mild forms of these conditions, such as upper airway resistance syndrome or respiratory effort related arousals, can be addressed before they begin to harm the patient. Because of the ability to influence growth in skeletally immature patients, orthodontic and dentofacial intervention can be instrumental in idealizing space appropriation and increasing OCV. However, this requires enlisting alternative orthodontic approaches such as SFOT and/or using temporary skeletal anchorage in rapid palatal expansion prior to the mid-palatal suture fusing. Temporary skeletal anchorage applied to rapid maxillary expansion can be used to achieve skeletal growth modification and avoid expansion at the dentoalveolar level, which may be detrimental to the periodontium or increase the incidence of relapse.42
Once a diagnosis has been made, IDT collaboration among the orthodontist, periodontist, and oral and maxillofacial surgeon can focus on engineering strategies to enhance the orthodontic walls.6,38 This optimization of dentoalveolar bone can then accommodate ideal tooth movement, expand overall OCV, overcome regional anatomic limits, and limit or avoid iatrogenic compromises. Depending on the outcome objective, SFOT may be adequate to accomplish these goals. However, OGS is required to address facial disharmonies and to definitively manage obstructive sleep-disordered breathing conditions.
When planning and executing IDT cases that require significant tooth movement, especially those designed to expand OCV, CBCT analysis is critical for assessing the condition and position of regional anatomy. 3D CBCT imaging offers a more complete picture of what may otherwise appear healthy or normal from a clinical examination.43 Further, 2-dimensional (2D) data often fail to accurately depict important anatomic structures that may be vital to comprehensive treatment planning for IDT patients.
Such collaborative interactions among multiple treatment team members require "restorative leadership" and demand that the primary-care dentist be well-versed in planning and executing a comprehensive treatment plan from a facially prioritized perspective. Because IDT imaging usually involves a large field of view CBCT for meaningful and comprehensive treatment planning, oversight and interpretation of the DICOM dataset by an oral and maxillofacial radiologist (OMFR) should be considered to optimize patient safety, provide medicolegal documentation, and allow inclusion of OMFR patient interpretation for the IDT team during treatment planning.
Even dentists who may be uncomfortable managing complex cases have a significant role in improving patient health. A patient's primary-care dentist is often the most frequent oral healthcare contact for the patient. The dentist is uniquely positioned to evaluate a worn or crowded dentition, assess sleep-related dental and airway anatomy, and make appropriate specialty referrals. CBCT and other applicable 3D modalities can be used to assess tongue volume and position in relation to total OCV.26,44-51 The presence of tongue crenations may be an indication that an OCV problem and possible undiagnosed sleep-disordered breathing condition are present.52,53 Screening questionnaires such as STOP-BANG and the Epworth Sleepiness Scale also can be used to gain insight into risk assessment.54 Any or all of these methods may help a primary-care dentist determine if follow-up is required for the patient.
Regional Acceleratory Phenomenon and SFOT
Corticotomy-based SFOT is able to accelerate tooth movement solely through the transient demineralization of the bone matrix. The orthodontic movement of teeth during this period is completely periodontal ligament mediated and dependent. As such, orthodontic principles of tooth movement are unchanged. However, these principles must be used with an understanding of the postsurgical physiology of the injured and augmented dentoalveolar bone. The use of anchor plates, temporary anchorage devices, and creative orthodontic mechanics can be considered during the RAP to maximize the advantage of the bone remodeling capabilities. Aligner therapy can be used either independently or in conjunction with conventional brackets and archwires to improve patient acceptance, compliance, and hygiene abilities.
The decision to perform corticotomy-assisted orthodontic surgery on the buccal, lingual, or both aspects of the tooth depends on: (1) the orthodontic engineering/plan, (2) where the demineralized bone matrix is required for effective tooth movement, and (3) the regional dentoalveolar bone anatomy. Accompanying bone augmentation surgery further allows the orthodontic boundary conditions to be expanded and optimizes conditions for facially prioritized treatment planning as well as periodontal/dentoalveolar bone phenotype outcomes.5 More clinical and histologic studies are needed to further validate SFOT in the management of IDT patients and better define the limits of tooth movement when dentoalveolar bone augmentation surgery is performed. While true periodontal regeneration on dehisced root surfaces has been shown in human histologic studies,5 the extent to which bone augmentation volume is gained and then maintained over time via a SFOT treatment model remains to be shown.
Completing the "Restoratively Driven Circle" in Comprehensive IDT
The outcome goal of SFOT and OGS is to achieve ideal root and tooth position in biologically sound locations within the orthodontic walls/boundary conditions, manage skeletal discrepancies, and achieve facial harmony. This sets the stage for creation of the most occlusally stable and proportionally correct dental rehabilitation.
When used for IDT in this manner, the primary objective of SFOT is health and stability as opposed to expeditious tooth movement. However, the overall reduction in orthodontic treatment time can indeed be critical for the patient who requires OGS in the context of general health management, especially in the presence of airway compromise such as OSA. Here, it can become urgent to expedite decompensations. Timely management of OSA is important to reduce the impact of its myriad systemic effects.55 SFOT can reduce the time required to prepare the patient for OGS without sacrificing the periodontium in the presence of a thin dentoalveolar bone phenotype. By decompensating and facilitating tooth movement in a demineralized bone matrix and augmented dentoalveolar bone environment, the IDT team can minimize or eliminate pre-OGS orthodontic compromises. Thus, the patient is positioned for life-altering OGS/skeletal surgery and its beneficial effects on airway volume (as well as facial harmony)56 as quickly as possible. Expedited treatment can also minimize the overall time spent in provisional restorations, reducing the opportunity for caries to develop or re-emerge.
At the completion of SFOT-inclusive IDT, the team will have addressed the buccal bone thickness, proper placement of the dentogingival complex (including physiologically and restoratively/esthetically optimized biologic width), and optimal root position within newly augmented alveolar bone. The relationships of teeth to teeth (dental), teeth to jaws (dentoalveolar deficiencies and alveoloskeletal discrepancies), jaws to jaws (intra-arch relationships), and jaws to face (dentofacial disharmonies) are optimized to maximize dental, periodontal, occlusal, and systemic health (airway) conditions. Such foundations ensure long-term sustainability of ideal function and facial esthetics that will meet the patient's expectations during the subsequent restorative phase. The teeth and smile can be rehabilitated using the most minimally invasive/bonded restorative therapies possible. Minimally invasive restorative/rehabilitative approaches can improve long-term outcomes and prognoses, offering the best opportunity to sustain the greatest number of vital teeth over the longest time.
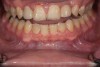
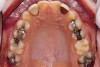
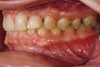
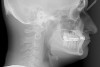
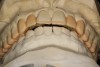
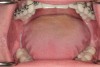
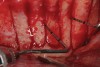
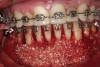
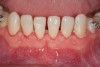
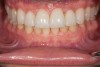
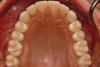
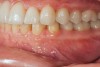
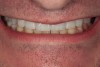
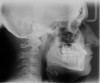
Figure 2 through Figure 17 demonstrate a case that was managed with SFOT-based IDT therapy under the conditions and with the rationale discussed in this article.
Conclusions
The paradigm shift from treatment planning that is occlusally based to a facially prioritized treatment plan requires that clinicians focus on the tooth, root, and soft-tissue positions. When treatment planning with a facially prioritized perspective that involves CBCT imaging and diagnoses, SFOT becomes an increasingly important instrument for clinicians. The IDT team needs to manage the dynamic craniomandibular system with greater totality. Through further research, the exact roles, indications, and efficacy of SFOT for this treatment may be elucidated.
Emphasis must be placed on changing the way that dentistry identifies and manages dentofacial deformities and dentoalveolar bone phenotypes so that patient results can be optimized. Effective communication among all team members is the initial step toward a meaningful cultural shift in the direction of a facially prioritized IDT collaboration. Education in IDT planning will likely require more training (or sub-specialization) as the scope of dentoalveolar surgical therapy expands. Finally, while technology has greatly improved dentistry's diagnostic acumen, it is not a substitute for experience or judgment and cannot replace the principles of biology or wound healing.
About the Authors
George A. Mandelaris, DDS, MS
Adjunct Clinical Assistant Professor, University of Illinois, College of Dentistry,
Department of Graduate Periodontics, Chicago, Illinois; Adjunct Clinical Assistant Professor, University of Michigan, School of Dentistry, Department of Graduate
Periodontics, Ann Arbor, Michigan; Private Practice, Periodontics and Dental
Implant Surgery, Chicago, Illinois
Bradley S. DeGroot, DDS, MS
Private Practice, Periodontics and Dental Implant Surgery, Chicago, Illinois
Robert Relle, DDS
Lecturer, University of California-Los Angeles, School of Dentistry, Department of Oral and Maxillofacial Surgery, Los Angeles, California; Private Practice, Oral and Maxillofacial Surgery, Los Angeles, California
Brian Shah, MD, DDS
Director of Research, Department of Oral and Maxillofacial Surgery, Yale New Haven Hospital, New Haven, Connecticut; Private Practice, Oral and Maxillofacial Surgery, Chicago, Illinois
Iwei Huang, DMD, MS
Private Practice, Orthodontics and Dentofacial Orthopedics, Chicago, Illinois
Brian S. Vence, DDS
Private Practice, Restorative Dentistry, Oakbrook Terrace, Illinois
Queries to the author regarding this course may be submitted to
authorqueries@aegiscomm.com.
References
1. Amsterdam M. Periodontal prosthesis. Twenty-five years in retrospect. Alpha Omegan. 1974;67(3):8-52.
2. Enlow DH, Moyers RE. Growth and architecture of the face. J Am Dent Assoc. 1971;82(4):763-774.
3. Ahn J, Kim SJ, Lee JY, et al. Transverse dental compensation in relation to sagittal and transverse skeletal discrepancies in skeletal Class III patients. Am J Orthod Dentofacial Orthop. 2017;151(1):148-156.
4. Staudt CB, Kiliaridis S. Different skeletal types underlying Class III malocclusion in a random population. Am J Orthod Dentofacial Orthop. 2009;136(5):715-721.
5. Mandelaris GA, Neiva R, Chambrone L. Cone-beam computed tomography and interdisciplinary dentofacial therapy: an American Academy of Periodontology best evidence review focusing on risk assessment of the dentoalveolar bone changes influenced by tooth movement. J Periodontol. 2017;88(10):960-977.
6. Handelman CS. The anterior alveolus: its importance in limiting orthodontic treatment and its influence on the occurrence of iatrogenic sequelae. Angle Orthod. 1996;66(2):95-109.
7. Braut V, Bornstein MM, Belser U, Buser D. Thickness of the anterior maxillary facial bone wall-a retrospective radiographic study using cone beam computed tomography. Int J Periodontics Restorative Dent. 2011;31(2):125-131.
8. Nowzari H, Molayem S, Chiu CH, Rich SK. Cone beam computed tomographic measurement of maxillary central incisors to determine prevalence of facial alveolar bone width ≥2 mm. Clin Implant Dent Relat Res. 2012;14(4):595-602.
9. Evensen JP, Ogaard B. Are malocclusions more prevalent and severe now? A comparative study of medieval skulls from Norway. Am J Orthod Dentofacial Orthop. 2007;131(6):710-716.
10. Krneta B, Zhurov A, Richmond S, Ovsenik M. Diagnosis of Class III malocclusion in 7- to 8-year-old children-a 3D evaluation. Eur J Orthod. 2015;37(4):379-385.
11. Joshi N, Hamdan AM, Fakhouri WD. Skeletal malocclusion: a developmental disorder with a life-long morbidity. J Clin Med Res. 2014;6(6):399-408.
12. Boyd K. Darwinian dentistry part 2: early childhood nutrition, dentofacial development and chronic disease. J Am Orthodontia Soc. 2012;12(2):28-32.
13. Agarwal SS, Sharma M, Nehra K, et al. Validation of association between breastfeeding duration, facial profile, occlusion, and spacing: a cross-sectional study. Int J Clin Pediatr Dent. 2016;9(2):162-166.
14. Kohli MV, Patil GB, Kulkarni NB, et al. A changing trend in eruption age and pattern of first deciduous tooth: correlation to feeding pattern. J Clin Diagn Res. 2014;8(3):199-201.
15. Thomaz EB, Cangussu MC, Assis AM. Maternal breastfeeding, parafunctional oral habits and malocclusion in adolescents: a multivariate analysis. Int J Pediatr Otorhinolaryngol. 2012;76(4):500-506.
16. Kim SJ, Kim KH, Yu HS, Baik HS. Dentoalveolar compensation according to skeletal discrepancy and overjet in skeletal Class III patients. Am J Orthod Dentofacial Orthop. 2014;145(3):317-324.
17. Qu X, Liu Z, Wang Y, et al. Dentofacial traits in association with lower incisor alveolar cancellous bone thickness: a multiple regression analysis. Angle Orthod. 2017;87(3):409-415.
18. Rose JC, Roblee RD. Origins of dental crowding and malocclusions: an anthropological perspective. Compend Contin Educ Dent. 2009;30
(5):292-300.
19. Gilbert SF. Ecological developmental biology: developmental biology meets the real world. Dev Biol. 2001;233(1):1-12.
20. Estelita S, Janson G, Chiqueto K. Extreme dental compensation in an adult skeletal class III malocclusion: 3-year follow-up of a successfully compromised treatment. Int J Orthod Milwaukee. 2015;26(2):
69-76.
21. Evangelista K, Vasconcelos Kde F, Bumann A, et al. Dehiscence and fenestration in patients with Class I and Class II Division 1 malocclusion assessed with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010;138(2):133.e1-e7.
22. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322.
23. Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):
1006-1014.
24. Patino M, Sadhasivam S, Mahmoud M. Obstructive sleep apnoea in children: perioperative considerations. Br J Anaesth. 2013;111(suppl 1):
i83-i95.
25. Nespoli L, Caprioglio A, Brunetti L, Nosetti L. Obstructive sleep apnea syndrome in childhood. Early Hum Dev. 2013;89(suppl 3):S33-S37.
26. Iida-Kondo C, Yoshino N, Kurabayashi T, et al. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imaging. J Med Dent Sci. 2006;53(2):119-126.
27. Iared W, Koga da Silva EM, Rufino Macedo C. Esthetic perception of changes in facial profile resulting from orthodontic treatment with extraction of premolars: a systematic review. J Am Dent Assoc. 2017;148(1):9-16.
28. Pliska BT, Tam IT, Lowe AA, et al. Effect of orthodontic treatment on the upper airway volume in adults. Am J Orthod Dentofacial Orthop. 2016;150(6):937-944.
29. Ferguson DJ, Makki L, Stapelberg R, et al. Stability of the mandibular dental arch following periodontally accelerated osteogenic orthodontics therapy: preliminary studies. Semin Orthod. 2014;20(3):
239-246.
30. Roblee RD, Bolding SL, Landers JM. Surgically facilitated orthodontic therapy: a new tool for optimal interdisciplinary results. Compend Contin Educ Dent. 2009;30(5):264-275.
31. Baloul SS, Gerstenfeld LC, Morgan EF, et al. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139(suppl 4):S83-S101.
32. Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65(1):79-83.
33. Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21(1):9-19.
34. Sebaoun JD, Kantarci A, Turner JW, et al. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008;79(9):1679-1688.
35. Merrill RG, Pedersen GW. Interdental osteotomy for immediate repositioning of dental-osseous elements. J Oral Surg. 1976;34(2):118-125.
36. Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983;31(1):3-9.
37. Zimmo N, Saleh MH, Mandelaris GA, et al. Corticotomy-accelerated orthodontics: a comprehensive review and update. Compend Contin Educ Dent. 2017;38(1):17-25.
38. Braut V, Bornstein MM, Lauber R, Buser D. Bone dimensions in the posterior mandible: a retrospective radiographic study using cone beam computed tomography. Part 1-analysis of dentate sites. Int J Periodontics Restorative Dent. 2012;32(2):175-184.
39. Mandelaris GA, Vence BS, Rosenfeld AL, Forbes DP. A classification system for crestal and radicular dentoalveolar bone phenotypes. Int J Periodontics Restorative Dent. 2013;33(3):289-296.
40. Yilmaz BS, Kucukkeles N. Skeletal, soft tissue, and airway changes following the alternate maxillary expansions and constrictions protocol. Angle Orthod. 2014;84(5):868-877.
41. Denolf PL, Vanderveken OM, Marklund ME, Braem MJ. The status of cephalometry in the prediction of non-CPAP treatment outcome in obstructive sleep apnea patients. Sleep Med Rev. 2016;27:56-73.
42. Evans M. Three-dimensional control with TAD-tissue supported rapid palatal expander: an overview of clinical applications and biological advantages. Orthodontic Clinical Review. 2013:22-31. https://www.rmortho.com/wp-content/uploads/2013/08/Clinical_Final_LR.pdf. Accessed January 24, 2018.
43. Gauthier C, Voyer R, Paquette M, et al. Periodontal effects of surgically assisted rapid palatal expansion evaluated clinically and with cone-beam computerized tomography: 6-month preliminary results. Am J Orthod Dentofacial Orthop. 2011;139(suppl 4):S117-S128.
44. Xiong G, Hu J, Chen W, et al. The analysis of correlation between tongue body MRI and upper airway pressure measurements of blocked lingual region in patients with moderate and severe OSAHS. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(21):1853-1856.
45. Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37(10):1639-1648.
46. Fregosi RF. Influence of tongue muscle contraction and transmural pressure on nasopharyngeal geometry in the rat. J Appl Physiol (1985). 2011;111(3):766-774.
47. Babademez MA, Yorubulut M, Yurekli MF, et al. Comparison of
minimally invasive techniques in tongue base surgery in patients with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2011;145(5):
858-864.
48. Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol (1985). 2006;101(5):13771385.
49. Stuck BA, Kopke J, Hormann K, et al. Volumetric tissue reduction in radiofrequency surgery of the tongue base. Otolaryngol Head Neck Surg. 2005;132(1):132-135.
50. Do KL, Ferreyra H, Healy JF, Davidson TM. Does tongue size differ between patients with and without sleep-disordered breathing? Laryngoscope. 2000;110(9):1552-1555.
51. Powell NB, Riley RW, Guilleminault C. Radiofrequency tongue base reduction in sleep-disordered breathing: a pilot study. Otolaryngol Head Neck Surg. 1999;120(5):656-664.
52. Zonato AI, Bittencourt LR, Martinho FL, et al. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2003;113(6):
973-980.
53. Zonato AI, Martinho FL, Bittencourt LR, et al. Head and neck physical examination: comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope. 2005;115(6):1030-1034.
54. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
55. Khayat R, Pleister A. Consequences of obstructive sleep apnea: cardiovascular risk of obstructive sleep apnea and whether continuous positive airway pressure reduces that risk. Sleep Med Clin. 2016;11(3):273-286.
56. Uesugi T, Kobayashi T, Hasebe D, et al. Effects of orthognathic surgery on pharyngeal airway and respiratory function during sleep in patients with mandibular prognathism. Int J Oral Maxillofac Surg. 2014;43(9):1082-1090.