You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Due to increased patient demands for esthetics and the high cost of gold in recent years, the number of all-ceramic systems on the dental market has proliferated. Zirconia-based ceramics have become one of the prime alternatives to metal-ceramic restorations. Zirconia is a metastable material that can exist in various crystalline phases, three types of which have been utilized for dentistry: tetragonal, monoclinic, and cubic.1 The first version of zirconia employed in dentistry, now in use for more than 10 years, is a form comprised of the high-strength tetragonal crystalline phase.2 At room temperature zirconia exists in the weaker monoclinic crystalline form3; however, small amounts of oxides, or dopants, are added to stabilize the tetragonal crystalline form.4 The main commercial version is stabilized with 3 mol% yttria and is called yttria-stabilized tetragonal zirconia polycrystal (3Y-TZP).3 Originally, 0.25% of alumina was also added because it aids in chemical stability of zirconia, minimizing the potential for low-temperature moisture degradation as might occur in the oral environment.3,5 Most brands of high-strength zirconia are enhanced with 0.25% of alumina.
The original high-strength zirconia, while having excellent physical properties and a white hue, is opaque and requires layering with porcelain in the same fashion as a porcelain-fused-to-metal restoration to obtain even reasonable esthetics.2 Tetragonal zirconia has a relatively high refractive index. The addition of alumina, with a different refractive index than zirconia, causes light passing through alumina-doped zirconia to be scattered or absorbed at grain boundaries enough to make it relatively opaque even in 1-mm thickness.2,6 Density of processing (ie, air pockets), particle size, and particle distribution also each play a role in the opacity of tetragonal zirconia.2
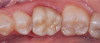
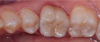
Dentistry’s elusive goal regarding use of all-ceramics is the fabrication of restorations with minimal or no application of a secondary phase while maintaining esthetics. In other words, the aim is to develop a monolithic material with optical properties closely resembling the natural tooth that can be used without layering of porcelain. High-strength glass-ceramics, such as lithium disilicate, have been developed in a monolithic form with very good optical properties and are used successfully for this purpose.7 In an initial attempt to produce a more translucent version of zirconia, developers reduced the alumina content from 0.25% to less than 0.05% and improved processing techniques to control zirconia grain size and processing density to minimize light refraction and increase translucency.2 Manufacturers now generally believe the alumina was not necessary for dental zirconia to prevent low-temperature degradation (phase transformation), because dental zirconia does not undergo the constant physical and chemical stresses that a joint implant does. It is possible that it was likely added only as a precaution, although the authors could not find any supportive research. Two commonly known brands of this generation of zirconia are BruxZir® (Glidewell Laboratories, glidewelldental.com) and Lava™ Plus (3M ESPE, 3m.com). These materials, while offering a slight increase in translucency compared with original zirconia, still lack the necessary optics to be used in the anterior but benefit from minimal microlayering of porcelain (Figure 1 and Figure 2). The reduction in alumina and improvement in processing technique has minimal effect on the mechanical properties of the material.3
The third and most recent strategy to increase the translucency of zirconia is to stabilize it with a significant cubic crystalline phase interspersed with the tetragonal phase. Increasing the yttria content to more than 8 mol% will stabilize the cubic phase.2 Several versions of “high-translucent” or “cubic-containing” zirconia have come on the market recently, manufactured with approximately 8 mol% yttria to 10 mol% yttria. Examples of these include: Lava™ Esthetic (3M ESPE); Katana™ Zirconia (UTML/STML) (Kuraray Noritake Dental Inc., kuraraynoritake.com); BruxZir®argen.com); and Imagine® (Jensen Corp., jensendental.com). The exact amount of cubic phase in these types of materials, though proprietary, ranges from 10% to 15%, according to manufacturers. The cubic phase of zirconia is isotropic in different crystallographic directions, which decreases light scattering that occurs at grain boundaries.2,8,9 As a result, the cubic zirconia appears more translucent.2,8,9 The translucency of dental polycrystalline cubic zirconia should not be confused with the complete transparency of cubic zirconia used in jewelry, which is a single-crystal structure (ie, no grain boundaries). Stabilized cubic zirconia does not transform at room temperature; therefore, cubic zirconia will not undergo transformation toughening or low-temperature degradation. In other words, it is more susceptible to mechanical damage,2,10 though it will not degrade over time. Generally, all materials on the market list their flexural strength at approximately 40% less than the high strength of all-tetragonal versions. Most manufacturers report their materials to be in the 600 MPa to 750 MPa range for flexural strength and claim that they have both the translucency and strength to be used for single restorations anywhere in the mouth.11
The purpose of this article is to examine some of the newer cubic zirconia materials used in a monolithic form. First, the authors will discuss laboratory-processing issues that affect esthetics, including evaluation of colorizing, sintering, finishing, and polishing. Second, the authors will assess initial translucency testing of several materials and evaluate the effect of air abrasion on flexural strength of these cubic zirconias. It has been well established in the literature that to increase the adhesion of zirconia, air abrasion with aluminum oxide is necessary.12 Published reports have shown no negative or weakening effects of high-strength zirconia with air abrasion and have demonstrated an increase in strength with some materials, probably due to a monoclinic crystal transformation.13 Known as transformation toughening, tetragonal zirconia “transforms” into monoclinic-form crystals, which are 3% to 5% larger. This gives it the ability to compress crack growth through localized volumetric expansion.4 Cubic zirconia will not undergo this change. No published reports could be found on the effects alumina air abrasion has on strength at pressures recommended for increasing adhesion with resin cements and special primers. Lastly, and perhaps most importantly, this article demonstrates an anterior single-unit monolithic case with several of the newer materials and contrasts it to what is considered to be a standard in monolithic esthetics, lithium disilicate.
Laboratory Considerations
That Affect Esthetics
In an extensive search of the literature and social media, the authors could not find any published information or recommendations (other than from a manufacturer) on how to “pre-finish” (either texturize or colorize) these new cubic zirconia materials for monolithic use. The authors considered it very important to laboratory-test colorization and texturization techniques both in presintering and postsintering stages to be able to make initial recommendations for how best to handle this material.
Cubic zirconia in its green state, or unsintered, grinds fairly similar to grinding on a block of hard chalk. When ground on, these new materials felt similar to original high-strength zirconia materials, although they seemed to be more brittle and chip easier at the margin when finishing; thus, considerable care needs to be taken in these regions. Texturizing the surface of cubic zirconia with different coarseness of carbides and laboratory diamonds gave very different results (Figure 3). In the authors’ hands and experience, fine diamonds used in an electric handpiece at approximately 10,000 RPM to 12,000 RPM resulted in the most natural-looking texture (Figure 4). After using the diamonds, the authors tested two different impregnated rubber polishers—one gray point and one pink point—with the latter used at approximately 8,000 RPM giving the most natural finish, in the authors’ estimation (Figure 5 and Figure 6).
The authors also attempted to integrate texture into postsintered cubic zirconia and then re-polish. They found that it was either impossible to maintain natural-looking surface texture or the surface was microrough after polishing, which could create an additional problem of abrasion against the opposing tooth.14 Therefore, the authors highly recommend putting all the form surface texture and prepolish into the restoration prior to sintering, regardless of the brand or type of zirconia used.
Some of the pigments from some of the manufacturers for colorizing and/or giving basic shade color to the cubic zirconia were found to be fairly low in chroma during application. This made it difficult for the authors to know if they had achieved their goal when applying an even coating or a specific pattern (Figure 7). The authors found that the Zirkonzahn pigments (zirkonzahn.com) had the best visible color during application and were the most user friendly (Figure 8). These pigments worked with all systems and provided somewhat similar results for each. Thus, if a clinician prefers a pigment from a specific system, it is possible to use it with another company’s zirconia of similar cubic/tetragonal phase relationship. One option to better visualize the application of the pigments or colorants the authors tested was to add food coloring (Figure 9 and Figure 10) to the pigments to intensify the visual color and see more clearly the amount applied. The food colorants were added only as a contrast media to better visualize the actual zirconia color pigment being applied. As long as the food colorant did not contain any form of sugar, it burned out cleanly. The authors used Kroger (kroger.com) food colorants. It is important to note that the manufacturers have no recommendation regarding this technique. The manufacturers provide specific guidance for how to apply their colorants; adding the food color to low visually chromatic pigments allows the applicator to see if the colorants have been applied in the desired pattern. This addition of food coloring did not cause any negative postsintering problems.
Based on the visual analysis of the evaluators (McLaren, Kang, Trujillo, and Choi), calibrated photography, and laboratory evaluation in Photoshop from a section from the middle one third of the shade guide and middle one third of the crown (Figure 11), the Argen colorants came closest to matching the A2 shade guide when using A2-labeled pigments, and Zirkonzahn pigments offered the most ability to custom colorize a restoration, which would be more of a need for anterior teeth.
The most significant laboratory concern was a sensitivity to firing temperature; a 30°C change either plus or minus caused a discernable difference in perceptible translucency of a molar full-contour crown (Figure 12 through Figure 14). Thus, a high-quality oven that has even firing parameters throughout the firing chamber is paramount for controlling esthetic success as it relates to obtaining desired translucency. The authors tried three ovens, which were calibrated per manufacturers’ recommendations. Several test crowns were fired at different heights within the containing tray as a test to determine if different zones in the oven had even heat. Only one oven (Zircom, KDF, kdfus.com) gave consistent results. This suggests that oven choice is a major consideration that is perhaps more critical than specific material with cubic zirconia at this early stage of development. The oven quality and correct firing was a critical aspect of the process and must not be overlooked. Once the material is sintered, chairside staining will not affect the translucent result of the crown other than by blocking it with pigments from the surface. The firing of surface pigments will not alter the structure physically or optically, unlike glasses.
The effect of the color of the preparation on the final perceived color was evaluated visually by fabricating a single molar crown that had a 1.2-mm thickness and was colorized as closely as possible to match the A2 shade of the Vita classical shade guide (VITA Zahnfabrik, vita-zahnfabrik.com). The crown was placed on five resin composite dies fabricated with 1M1 to 5M3 shades based on the Vita classical shade system (Figure 15). A significant difference can be seen in the gingival one third of the restoration, which is due to “bleed through” of the color of the preparation. This would need to be considered in the shade-matching process. For this article, no attempt was made to quantify the amount of color change due to the substrate; however, this will be the topic of future research.
One last important observation by the authors relative to obtaining ideal esthetics has to do with glaze and polish. In their judgment, glazing alone did not result in a realistic surface as it might with normal porcelain (Figure 16, right half of tooth). Rather, mechanical polishing with Dialite polishers (Brasseler USA, brasselerusadental.com) and then diamond polishing paste appeared to give a more realistic finish to the surface (Figure 16, left half of tooth). Viewing conditions were done in a light box with even illumination set at 5500 degrees Kelvin.
Translucency and Effects of Air Abrasion Testing
Translucency Testing
Manufacturer-fabricated specimens were tested for translucency. The main requirements were that the specimens be 1 mm in thickness and at least 10 mm in diameter, or square. On arrival, the authors photographed the specimens with a white background and a black line to visually evaluate translucency (Figure 17). Visual inspection revealed that the cubic zirconia A2 specimens were similar in translucency—that is, slightly visually less translucent—to the e.max MT (medium translucency) (Ivoclar Vivadent, ivoclarvivadent.us) and significantly less translucent than the e.max HT (high translucency) (Ivoclar Vivadent). All specimens were A2, but each had a different appearance. (Note: The authors are planning a more exhaustive test with exact uniform specimens to test transmission with a transmission spectrometer.)
Flexural Strength Testing
The three-point bend flexural strength of a representative sample of translucent (ArgenZ Anterior [Argen]; DD CubeX2 [Dental Direkt, dentaldirekt.com]; Prettau® Anterior® [Zirkonzahn]; BruxZir® Anterior [Glidewell Laboratories]; Jensen HT [Jensen Dental]) and traditional (ArgenZ Esthetic [Argen]; DD Bio ZX2 [Dental Direkt]) zirconia materials were tested according to ISO 6872. Specimens were prepared by sectioning the zirconia into bars measuring 25 mm x 4 mm x 2 mm, sintering according to manufacturers’ recommendations, and polishing all specimens with 1,200-grit abrasive paper. Specimens (N = 5) were then prepared according to three treatment conditions: (1) control with no modification; (2) particle abrasion at 2-bar (30 psi) pressure; and (3) particle abrasion at 4-bar (60 psi) pressure. All particle abrasion was performed with 50-µm alumina for 10 seconds from a distance of 10 mm. The specimens were placed in a universal testing machine on 20-mm separated supports and loaded to failure at 1 mm/min (Figure 18). The maximum failure load was used to calculate the flexural strength. A representative specimen of both traditional and translucent zirconia was treated with 2-bar alumina particle abrasion and observed with a scanning electron microscope.
The results of this pilot study (Table 1) indicated that traditional zirconia does not have a significant decrease in strength after particle abrasion, and one traditional zirconia material actually became stronger after particle abrasion. This observation is not unique, as previous studies have shown improvements in flexural strength after particle abrasion.13 The increase in strength after particle abrasion is presumed to occur following transformation of tetragonal zirconia to monoclinic zirconia in transformation toughening. Following 2- and 4-bar particle abrasion, the translucent zirconia tested in the study showed a significant decrease in strength. The translucent zirconia contains zirconia in its cubic phase, which does not transform, and therefore will not undergo transformation toughening following particle abrasion. The surfaces of the traditional and translucent zirconia following particle abrasion showed nearly identical surface damage (Figure 19 and Figure 20). The similarity of the surfaces provides evidence that the difference in behavior of the traditional and translucent zirconia is due to a lack of transformation toughening with translucent zirconia.
No clinical conclusions can be drawn from these data, and the authors are investigating different surface treatments that may not detrimentally affect the strength of cubic-phase–containing zirconia, such as less abrasive particles and lower air pressure. These treatments, in combination with proven zirconia primers (ie, materials containing 10-methacryloyloxydecyl dihydrogen phosphate [MDP]), may enable durable bonding to cubic-phase–containing zirconias.15 However, it should not be assumed that because this is zirconia it can be used in a reduced thickness without adhesion for posterior crowns. Another assumption is that it would behave clinically the same as the all-tetragonal-phase zirconia. No evidence supports this claim, and clearly it is much easier to damage or weaken cubic-containing zirconia, which already starts with a lower strength. The authors’ preliminary recommendation for bonding to cubic-containing zirconia is to use a system that utilizes 30-µm alumina particles coated in silica followed by a primer containing silane and MDP (eg, Monobond Plus [Ivoclar Vivadent]; Clearfil™ Ceramic Primer [Kuraray]). One benefit of adhesion is increased stress distribution, which theoretically should minimize crack initiation and crack growth and is the basic reason bonded porcelain works.
Case Evaluation
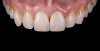
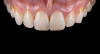
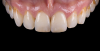
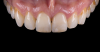
For clinical evaluation of several commercial systems, a single central crown case was chosen, because single centrals are generally regarded as the biggest esthetic challenge in dentistry. A case was selected with a normal color substrate (Figure 21) with a facial reduction of approximately 1.2 mm. While this article is not intended to comment on all aspects of patient treatment, it should be noted that when taking shade information after preparation the teeth should be hydrated because teeth will appear brighter when they are dehydrated. Noritake STML (Kuraray Noritake Dental Inc.), ArgenZ Anterior (Argen), CubeX2 (Dental Direkt), and Jensen Imagine (Jensen Dental) were fabricated using a monolithic technique and compared to e.max MT as a control. Presintering colorization was performed using manufacturer-supplied colorants. As stated earlier, to custom colorize, the presintered cubic zirconia required custom application of shade colorants and shade modifiers to match the adjacent natural tooth. This clearly would require experience with a given system to know how the main shade colors behave and how to use custom modifiers. Therefore, an experienced ceramist performed the task.
For the Noritake STML, the material is already internally colorized and has multilayered color with both incisal and dentin colors. A slightly brighter color was chosen than the central to be matched knowing that a surface application of color would be necessary to “fine tune” the color match. Shade A2 of e.max was used as the standard monolithic esthetic control. All systems required two coats of external colorants and separate firings to obtain the intricate color of the matched natural tooth. As described in the laboratory section earlier, all systems were then glazed with an overglaze and mechanically polished to obtain natural surface finish and gloss. Figure 22 through Figure 25 demonstrate the esthetic results of the four systems (Noritake STML, ArgenZ Anterior, CubeX2, and Jensen Imagine, respectively), while Figure 26 depicts the e.max MT. The images clearly show that an excellent esthetic result was obtained with monolithic cubic zirconia, similar in appearance and esthetic value to that of the control.
Conclusion
Cubic-containing zirconia behaves differently to air abrasion than the original high-strength tetragonal zirconia without a cubic phase. Thus, one should be cautious making clinical decisions based on research and experience obtained with tetragonal zirconia. In the opinion of the authors, cubic-containing zirconia is very sensitive to proper laboratory processing techniques, thus highly controlled processes have to be followed to obtain the mechanical and esthetic properties reported. Clinical evaluation on monolithic cubic-containing zirconia showed excellent promise as an esthetic restoration.
References
1. Howard CJ, Hill RJ. The polymorphs of zirconia: phase abundance and crystal structure by Rietveld analysis of neutron and X-ray diffraction data. J Mater Sci. 1991;26(1):127-134.
2. Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014;30(10):1195-1203.
3. Tong H, Tanaka CB, Kaizer MR, Zhang Y. Characterization of three commercial Y-TZP ceramics produced for their high-translucency, high-strength and high-surface area. Ceram Int. 2016;42(1 Pt B):1077-1085.
4. Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24(3):299-307.
5. Matsui K, Ohmichi N, Ohgai M, et al. Effect of alumina-doping on grain boundary segregation induced phase transformation in yttria-stabilized tetragonal zirconia polycrystal. Journal of Materials Research. 2006;21(9):2278-2289.
6. Zhang H, Li Z, Kim BN, Morita K, et al. Effect of alumina dopant on transparency of tetragonal zirconia. Journal of Nanomaterials. 2012;2012(2012):1-5.
7. Sulaiman TA, Delgado AJ, Donovan TE. Survival rate of lithium disilicate restorations at 4 years: A retrospective study. J Prosthet Dent. 2015;114(3):364-366.
8. Harada K, Raigrodski AJ, Chung KH, et al. A comparative evaluation of the translucency of zirconias and lithium disilicate for monolithic restorations. J Prosthet Dent. 2016;116(2):257-263.
9. Peuchert U, Okano Y, Menke Y, et al. Transparent cubic-ZrO2 ceramics for application as optical lenses. Journal of the European Ceramic Society. 2009;29(2):283-291.
10. Lucas TJ, Lawson NC, Janowski GM, Burgess JO. Effect of grain size on the monoclinic transformation, hardness, roughness, and modulus of aged partially stabilized zirconia. Dent Mater. 2015;31(12):1487-1492.
11. Zhang F, Inokoshi M, Batuk M, et al. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater. 2016;32(12):e327-e337.
12. Yang B, Barloi A, Kern M. Influence of air-abrasion on zirconia ceramic bonding using an adhesive composite resin. Dent Mater. 2010;26(1):44-50.
13. Ozcan M, Melo R, Souza RO, et al. Effect of air-particle abrasion protocols on the biaxial flexural strength, surface characteristics and phase transformation of zirconia after cyclic loading. J Mech Behav Biomed Mater. 2013;20:19-28.
14. Lawson NC, Janyavula S, Syklawer S, et al. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J Dent. 2014;42(12):1586-1591.
15. Kwon SJ, Lawson N, Beck P, et al. Bond strength, wear, and enamel wear of translucent zirconia [abstract]. J Dent Res. 2016;95(spec iss A): Abstract 0244.
This article was double-blind peer reviewed by members of IDT’s Editorial Advisory Board
About the Authors
Edward A. McLaren, DDS, MDC
Professor, Co-Director
Division of Biomaterials
Department of Clinical and Community Sciences UAB School of Dentistry
Birmingham, Alabama
Nathaniel Lawson, DMD, PhD
Assistant Professor
Co-Director
Division of Biomaterials
Department of Clinical and Community Sciences
UAB School of Dentistry
Birmingham, Alabama
James Choi
Co-Director
UCLA Master Dental Ceramist Program
Director Esthetic Dentistry Laboratory
UCLA School of Dentistry
Los Angeles, California
Juan Kang
Master Ceramist
Assistant Director of Esthetic Dentistry Laboratory
UCLA School of Dentistry
Los Angeles, California
Carlos Trujillo, DDS
Senior Fellow
UCLA Advanced Esthetics and Restorative Dentistry
UCLA School of Dentistry
Los Angeles, California