You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Seventeen years ago, as a student of Peter Dawson, DDS, this author first observed in his patients that the elimination of occlusal interferences enabled many of them to open and close their jaws faster, move more freely through excursive jaw movements, and feel more comfortable. Consequently, the author was motivated to investigate the causes of these phenomena by exploring the scientific literature. Research demonstrated that, while an enormous volume of relevant scientific publications existed, the scientific basis for occlusal therapy remained unclear. To understand occlusal therapy’s success, information would have to be integrated from widely divergent fields, such as neurophysiology, biomechanics, and histology, and individual interdisciplinary research protocols designed. This article encapsulates the findings to date.
Mechanoreception
Teeth are specialized organs that function to nourish and sustain life. While people eat, the brain rapidly compares food’s texture and hardness in the mouth to previous encounters and determines the best chewing strategy. Optimal chewing forces and rhythms are developed based on tactile sensory feedback from the food bolus’s contact with the teeth and soft tissues as the bolus progressively becomes smaller. The ability of a tooth to endure the rigors of mastication depends on having a durable stone-like structure and a complex neural control system to maintain the tooth’s integrity. The cornerstone of this neural control system is an exquisitely sensitive network of mechanoreceptors within the tooth and its periodontal ligament. Dental mechanoreceptors play a crucial role in providing tactile sensory feedback that minimizes the stresses that teeth endure while they pulverize vast quantities of food in a lifetime. Under the influence of pathologic conditions such as malocclusion or central nervous system disease, the teeth’s mechanosensory system can play a key role in promoting destructive oromotor behaviors, such as bruxism and clenching.
Mechanoreception is the unconscious sensing or conscious perception of touch or mechanical displacement arising from stimuli outside the body. Mechanoreceptors are sensory end organs that respond to mechanical stimuli such as tension, pressure, or vibration.
Perception and recognition of a finely textured object that is handled or bitten relies on the ability to encode tactile cues arising from its size, shape, and roughness. The encoding of these cues occurs primarily as a result of two types of mechanoreceptors, which include slowly adapting (SA) and rapidly adapting (RA) mechanoreceptors. SA mechanoreceptors, such as Merkel disks and Ruffini endings, fire continuous streams of action potentials as long as the stimulus (eg, touch) remains active. Because they fire continuously during contact, SA mechanoreceptors are best suited for providing awareness that an object is between the teeth.
Vibrations are produced when textured objects rub against the surfaces of the skin or teeth. RA mechanoreceptors, such as Meisner and Pacinian corpuscles, fire briefly upon initiation of vibrating or rapidly accelerating stimulation, stop quickly, and are able to re-fire rapidly in response to a new stimulus. The rapid on/off firing characteristics of RA mechanoreceptors make them well suited for sensing the vibrations associated with textural assessment.
Historically, the tactile sensory function of the teeth had been ascribed solely to periodontal mechanoreceptors and pain perception to the richly innervated tooth pulp. However, studies indicate mechanoreceptors within teeth play an important role in their tactile sensory function. In 1955 a study by Lowenstein and Rathkamp compared tactile sensory thresholds of nonvital (ie, root canal treated teeth) to vital teeth and found tactile thresholds of nonvital teeth were 57% higher than those of contralateral vital teeth.1 The authors concluded that a specialized mechanosensory mechanism within the teeth contributed to tactile sensory function. In 1975 Linden2 failed to identify significant differences in the thresholds of vital and nonvital teeth. Although the method of tooth stimulation used by Linden was quite different than Lowenstein and Rathkamp’s, Linden’s study convinced the scientific community to support the hypothesis that periodontal receptors served as the principal receptors involved in dental mechanosensation.3
Once thought to contain only A-delta and C pain nerve fibers (ie, nociceptive), physiologic investigations have discovered the tooth pulp contains numerous rapidly conducting A-beta mechanoreceptive fibers.4 Dong and Chudler5 used electrophysiologic recording techniques in cats to measure the elapsed time for impulses to travel up the neural axis from stimulated intradental nerves through the brainstem and thalamus to the somatosensory cortex. They determined some intradental nerves conveyed mechanosensory information to the somatosensory cortex much faster than A-delta pain fibers. These intradental nerves were classified as A-beta mechanoreceptive fibers based on their rapid conduction velocities. Similar results have been repeated in monkeys6 and more recently in humans in whom Ab pulpal nerves have been mapped to a specific location in the somatosensory cortex, effectively adding mechanoreceptive pulpal nerves to the classic “sensory homunculus”7 first described by Penfield and Jasper.8
Studies in cats have shown the neurophysiologic properties of intradental and periodontal mechanoreceptors are functionally different.9,10 Rubbing sandpaper with different grit sizes on canine teeth causes frequency-encoded discharge patterns to arise in trigeminal ganglion neurons. These discharge patterns are unique to grit size, indicating that intradental mechanoreceptors are able to encode mechanical vibrations. Intradental mechanoreceptors have rapidly adapting response characteristics and encode vibrations throughout a wide frequency range. Periodontal mechanoreceptors have slowly adapting response characteristics and encode only lower vibration frequencies (Figure 1). Intradental mechanoreceptors respond to forces applied to the tooth from all directions (ie, omnidirectional) whereas periodontal mechanoreceptors respond only when forces are applied from specific directions (ie, unidirectional). In monkeys, periodontal mechanoreceptors are more numerous around the anterior teeth than the posterior teeth,11 which may partly account for reports that indicate tactile thresholds of anterior teeth are lower than those of posterior teeth.12
Vibration perception through human skin is essential for accurate perception of textured objects that are grasped.13 Similarly, vibration perception through the teeth enables accurate assessment of textured objects placed in the mouth. The author has developed a test to assess vibration perception thresholds of human teeth. The results show intradental mechanoreceptors encode vibrotactile tooth stimulation at amplitudes low enough to help discern textural differences in objects.14-17 These experiments demonstrate vital maxillary and mandibular incisors encode vibrations between 10 Hertz (Hz) and 315 Hz at low amplitudes and endodontically treated teeth lack the ability to encode vibrations.
The author’s research confirms the presence of intradental mechanoreceptors and suggests endodontic procedures may limit patients’ abilities to perceive vibrations associated with textural assessment of objects with their teeth. In addition, the results show vibration perception thresholds are related to stimulation frequency, suggesting the conflicting results of earlier studies by Lowenstein and Rathkamp1 and Linden2 may be attributed to the different vibration frequencies delivered by their respective tooth stimulation methods.
Having lost intradental mechanoreceptors, nonvital teeth may unwittingly allow the use of stronger-than-normal biting forces. Eventually, elevated occlusal forces may lead to tooth wear and catastrophic fractures in nonvital teeth. This hypothesis is supported by the excessively high fracture rate associated with nonvital teeth.18
Sensory Motor Integration
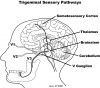
Sensory motor integration is a feedback process during which sensory inputs from peripheral parts of the body modify actions initiated by the central nervous system. This process occurs primarily in the brainstem, thalamus, and cortex (Figure 2). In the masticatory system, sensory motor integration coordinates fundamental activities such as breathing, eating, and swallowing with sensations that arise during their performance.
Occlusion of the jaws and teeth, as defined in The Glossary of Prosthodontic Terms, is “the act or process of closure.”19 Occlusion is a dynamic process during which willful and rhythmic jaw movements are integrated with sensations experienced during movement and memories of prior movements. Efferent motor commands from the cortex, cerebellum, and brainstem are integrated with peripheral sensory feedback from the teeth, muscles, temporomandibular joints, bones, and soft tissues. Occlusion relies on sensory motor integration to coordinate the activities of the muscles of mastication.
Movement coordination in most of the body’s joint systems (eg, arm and leg) is facilitated by proprioceptors (eg, muscle spindles) in antagonist muscle groups (ie, abductor and adductor muscles) and sensory receptors in the skin and joints. The masticatory system is unique in that only its adductor muscles (ie, jaw-closing muscles) are innervated by muscle spindles.20,21 As a result of this unique neural architecture, control of the jaw-opening muscles may be more reliant on tactile sensory feedback from mechanosensory receptors (ie, intradental and periodontal mechanoreceptors) than in other joint systems.
Mechanical tooth contacts produce very rapid jaw reflex behaviors. Jaw reflexes are thought to protect the teeth from excessively strong biting forces. Whether tooth contacts induce inhibition or excitation of the jaw-closing muscles depends on several variables, including rate of force application and background clenching level.22 Reflex inhibition of the jaw-closing muscles after mechanical tooth stimulation may be referred to as the jaw-opening reflex or silent period. In humans, the jaw-opening reflex is characterized by the rapid inhibition of the jaw-closing muscles (ie, masseter, temporalis, and medial pterygoid) and bite-force reduction, following tooth contact. When an unexpectedly high biting force occurs (eg, a stone in lentil soup) the jaw-opening reflex may prevent tooth fracture by rapidly shutting down the jaw-closing muscles.
Olgart et al23 monitored the jaw-opening reflex in cats in response to bending forces applied to their canine teeth. Their results demonstrated that bending forces applied to vital teeth evoke the jaw-opening reflex and subsequent endodontic procedures abolish this reflex. In conclusion, Olgart et al speculated a specialized sensory transducer mechanism exists in dentin that is activated by deformation or bending of the crown of a tooth.
Trulsson and Gunne observed “striking disturbances in the control of certain jaw motor behaviors” in people lacking dental mechanoreceptors.24 Participants with dentures and implants could not position their jaws as precisely as participants with vital teeth and used four times the biting force to hold a peanut between their teeth. These findings imply implant and denture prostheses are likely to undergo mechanical damage as a consequence of poor biting control.
RA intradental and SA periodontal mechanoreceptors generate streams of mechanosensory information as the teeth are maneuvered past each other during excursive jaw movements. Sensory motor integration of mechanosensory information from these receptors regulates the course and speed of the excursive movement. Patients with canine and incisive guidance have fewer contacting teeth during excursions and have less mechanosensory information to integrate than patients with group function and nonworking side interferences. Increasing the number of interfering tooth contacts during excursions compels the central nervous system to integrate more mechanosensory information because additional midcourse corrections are needed to accomplish the movement.
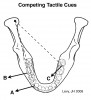
Posterior occlusal interferences compete with canine tooth contacts for control of masticatory muscles during excursive jaw movements (Figure 3). Competition for jaw-closing muscle activity occurs when posterior-interfering tooth contacts evoke muscle recruitment patterns that differ from those initiated by working-side anterior tooth contacts. Occlusal tooth contact competition can induce muscle hyperactivity in the orofacial region as jaw muscles become overworked. This may cause pain in these muscles. Reduced competition for muscle recruitment may explain why the elimination of working- and nonworking-side occlusal interferences can increase the speed of lateral jaw excursions, reduce muscle hyperactivity, and alter bruxing behaviors.25,26
A Paradigm Shift in Dentistry
Kuhn applied the term paradigm to the evolution of science. Kuhn wrote, “…a paradigm is an accepted model or pattern.…The new paradigm implies a new and more rigid definition of a field.…In the absence of a paradigm or some candidate for paradigm, all of the facts that could possibly pertain to the development of a given science are likely to seem equally relevant.”27 Controversies that shroud the field of occlusion may be resolved in time as irrelevant facts are pared away by the acquisition of new paradigms.
Teeth as sensory organs is a new paradigm in dentistry. In this paradigm, tooth contacts are understood to initiate streams of mechanosensory information that shape oromotor behavior. Endodontically treated teeth and dental implant-retained prostheses provide less mechanosensory information than vital teeth. It becomes clear that bite force magnitude is affected by mechanosensory feedback that can restrain muscle activity and limit structural damage to the teeth, temporomandibular joints, and periodontal apparatus. The function of occlusal therapy can be interpreted as the manipulation of mechanosensory streams to change jaw muscle activity and oromotor behavior. The goal of such therapy is to foster changes in oromotor behavior that reduce functional occlusal forces and positively affect the health and longevity of the masticatory system.
In the future, the application of the paradigm teeth as sensory organs may alter prosthetic treatment plans. Strategies may be developed that incorporate the fact that dental implants and nonvital teeth are more likely to be exposed to higher bite force levels because they are deficient in protective mechanosensation. Bias toward conservative tooth preparation may increase with widespread knowledge of how operative procedures affect intradental mechanoreception and vital teeth’s abilities to protect themselves from adverse biting forces.
References
1. Loewenstein WR, Rathkamp R. A study on the pressoreceptive sensibility of the tooth. J Dent Res. 1955;34(2):287-294.
2. Linden RWA. Touch thresholds of vital and non-vital human teeth. Exp Neurol. 1975;48:387-390.
3. Dubner R, Sessle BJ, Storey AT. The Neural Basis of Oral and Facial Function. New York, NY: Plenum Press; 1978:159.
4. Cadden SW, Lisney SJW, Matthews B. Threshold to electrical stimulation of nerves in cat canine tooth pulp with Ab, Ad, and C-fiber conduction velocities. Brain Res. 1983;26:31-41.
5. Dong WK, Chudler EH. Origins of tooth pulp-evoked far-field and early near-field potentials in the cat. J Neurophysiol. 1984;51(5):859-889.
6. Chudler EH, Dong WK, Kawakami Y. Tooth pulp-evoked potentials in the monkey: cortical surface and intracortical distribution. Pain. 1985;22(3):221-233.
7. Kubo K, Shibukawa Y, Shintani M, et al. Cortical representation area of human dental pulp. J Dent Res. 2008;87(4):358-362.
8. Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little, Brown & Company; 1954.
9. Dong WK, Chudler E H, Martin RF. Physiological properties of intradental mechanoreceptors. Brain Res. 1985;334(2):389-395.
10. Dong WK, Shiwaku T, Kawakami Y, et al. Static and dynamic responses of periodontal ligament mechanoreceptors and intradental mechanoreceptors. J Neurophysiol. 1993;69(5):1567-1582
11. Byers MR, Dong WK. Comparison of trigeminal receptor location and structure in the periodontal ligament of different types of teeth from the rat, cat, and monkey. J Comp Neurol. 1989;279(1):117-127.
12. Coffey JP, Williams WN, Turner GE, et al. Human bite force discrimination using specific maxillary and mandibular teeth. J Oral Rehabil. 1989;16(6):529-536.
13. Brisben AJ, Hsiao SS, Johnson KO. Detection of vibration transmitted through an object grasped in the hand. J Neurophysiol. 1999;81(4):1548-1558.
14. Robertson LT, Levy JH, Petrisor D, et al. Vibration perception thresholds of human maxillary and mandibular central incisors. Arch Oral Biol. 2003;48(4):309-316.
15. Levy JH, Robertson LT, Lilly DJ, et al. Possible role of intradental afferents in the mechanoreception of tooth contacts in humans. J Dent Res. 2002;81(spec iss A):3199.
16. Petrisor D, Levy JH, Robertson LT. Tactile thresholds of human maxillary and mandibular incisors. J Dent Res. 2002;81(spec iss A):3200.
17. Levy JH, Robertson LT, Lilly DJ, et al. Low frequency vibration thresholds of human maxillary central incisors. J Dent Res. 2003;82(spec iss A):1110.
18. Aquilino SA, Caplan DJ. Relationship between crown placement and the survival of endodontically treated teeth. J Prosthet Dent. 2002;87(3):256-263.
19. The glossary of prosthodontic terms. J Prosthet Dent. 2005;94(1):10-92.
20. Lennartsson B. Muscle spindles in the human anterior digastric muscle. Acta Odontol Scand. 1979;37(6):329-333.
21. Kubota K. Muscle spindle supply to the human jaw muscle. J Dent Res. 1977;56(8):901-909.
22. Yang J, Türker KS. Jaw reflexes evoked by mechanical stimulation of teeth in humans. J Neurophysiol. 1999;81(5):2156-2163.
23. Olgart L, Gazelius B, Sundström F. Intradental nerve activity and jaw-opening reflex in response to mechanical deformation of cat teeth. Acta Physiol Scand. 1988;133(3):399-406.
24. Trulsson M, Gunne HS. Food-holding and -biting behavior in human subjects lacking periodontal receptors. J Dent Res. 1998;77(4):574-582.
25. Kerstein RB. Disclusion time measurement studies; Part 2: A comparison of disclusion time length of 49 chronic myofascial pain dysfunction syndrome patients to 40 non-patients. A population analysis. J Prosthet Dent. 1994;72(5):473-480.
26. Trovato F, Orlando B, Bosco M. Occlusal features and masticatory muscles activity. A review of electromyographic studies. Stomatologija. 2009;11(1):26-31.
27. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962: 15-23.
About the Author
Jay Harris Levy, DDS
Private Practice, Portland, Oregon