You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Orthodontic treatment is one of the most conservative, long-lasting treatments to improve the esthetics and function of a patient. Bleaching is also one of the most conservative treatments to change the color of the patient’s teeth. Together, orthodontics and bleaching afford some of the most conservative, long-lasting treatment to offer a patient. Often, bleaching may follow orthodontic treatment, and occasionally use the orthodontic positioner as the tray with which to deliver the bleaching material. The most popular form for tray bleaching of the teeth involves the use of 10% carbamide peroxide in a custom-fitted tray.1
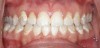
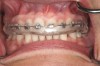
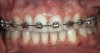
One of the most disappointing seque¬lae of orthodontic treatment may occur after the appliances are removed. Some¬times, white-spot lesions are present due to inadequate cleaning of the appliances during the 1- to 3-year treatment period (Figure 1). Some home care of orthodontic patients, especially teenagers, has been so obviously poor that the orthodontist has found it necessary to remove the braces before the completion of treatment to save the teeth from decay. The challenge of orthodontic treatment is to maintain the cleanliness of the braces throughout the treatment phase.
While bleaching will whiten teeth, tray bleaching with 10% carbamide peroxide has the side effect of removing plaque from teeth, improving gingival scores, and elevating the pH of the mouth and tray.2-8 Carbamide peroxide has been shown to kill many of the bacteria that cause tooth decay, as well as remove surface staining. This beneficial side effect affords a practical option to deal with the problems of oral hygiene during orthodontic treatment.
There have been many attempts to combine the properties of bleaching with the challenge of cleaning orthodontic patients. In the early 1960s, carbamide peroxide that was available over-the-counter (OTC) was used as a mouthwash in orthodontic patients for this reason, but with limited success, possibly due to the low contact time. When traditional nightguard vital bleaching was introduced in the late 1980s, fabrication of a custom-fitted tray over the brackets in the traditional method using an alginate impression and vacuum-formed matrix was determined to work better. However, over the course of the 1 to 3 years of orthodontic treatment, this approach would involve multiple impressions and trays as the teeth move every few months such that the previous tray would no longer fit the arch. Also, the main OTC ingredient with the best physical properties (Proxigel, GlaxoSmithKline Consumer Health Care, www.gsk.com) was removed from the market, leaving less desirable products available for this situation.
More recently, disposable trays with hydrogen peroxide to be worn for 30 to 60 minutes have been introduced as a cost-effective proposal for in-office debridement of the braces before the orthodontic visit. However, these trays do not fit well, and the nature of hydrogen peroxide does not retain its activity long enough to be beneficial in the caries control process, nor does the pH become elevated above that point at which tooth decay can occur. What is needed is a cost-effective method to create custom-fitted trays that can be worn overnight and contain a cost-effective carbamide peroxide and can be used for the duration of the orthodontic treatment to clean the braces of plaque and avoid white-spot lesions post-treatment. The purpose of this article is to present a technique that addresses those concerns by combining information from several sources in the bleaching literature with clinical applications.
Tray Fabrication
The traditional method for tray fabrication in the tray bleaching process involves a well-made alginate impression of the arch to be bleached. A stone cast is generated from this impression, and trimmed in such a manner as to work well in the vacuum former. The custom-fitted tray is formed from thin soft material.
When considering how to clean orthodontic braces using bleaching tray materials, the main missing portion of the oral hygiene puzzle has been a cost-effective tray fabrication technique that could be used multiple times during treatment. While the traditional alginate impression over the brackets was initially used, it was very difficult to obtain a good impression especially of the area of the teeth between the brackets and the gingiva. This area is the most difficult to clean, and yet the tray fits the poorest in this area. Additionally, the time and labor costs to remove the wires, make the alginate impression, pour the impression in cast stone, trim the cast, then fabricate a bleaching tray in a vacuum former for the many times this would be needed make that approach weary for the patient and the orthodontist.
An alternate method for bleaching normal teeth to the traditional impression, cast, and laboratory fabrication of trays is to use a thermoplastic tray formed directly in the mouth. A dual technique has been previously reported.9 A later development to this approach was the introduction of a single clear tray sold directly to dentists (Sure-Fit Ultra-Thin Professional Trays, Oratech, LLC, www.oratech.com; Ultra-Thin Dental Trays, Archtek, Inc, www.archtekinc.com). In this technique, the single clear soft tray is heated and softened in warm water that has been initially brought to a boil, then applied to the arch and directly contoured to the teeth by finger pressure. The patient then occludes into the softened tray and applies suction to form-fit the tray to the teeth. After the tray has cooled, the tray handle is then removed and the tray trimmed to fit. The use of this tray eliminates the impression stage for patients who may not tolerate impressions (those who might gag or choke using an alginate impression technique), and is useful in locations where laboratory equipment like a model trimmer or vacuum-forming machine is not available. Generally, a microwave oven, a coffee cup, and a pair of scissors are all that is needed to fabricate the tray. Occasionally, thermoplastic trays may not be long enough to completely cover the molars. However, it has been shown that 10% carbamide peroxide is effective as a bleaching agent well beyond the borders of the tray,10 and one might expect that the antimicrobial effects would extend beyond the tray as well.
The recently introduced thermoplastic trays, also called “boil and form” bleaching trays, were subsequently used with orthodontic patients to avoid removal of wires and multiple laboratory procedures. Those trays can be fabricated over the orthodontic braces directly in the mouth without removing wires or bands. Also, even though the trays are thermoplastic, they do not get soft enough to imbed themselves in the brackets, yet they can be readily adapted to the gingival area below the brackets, which is the hardest to clean.
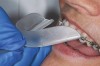
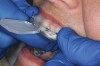
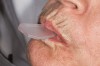
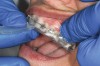
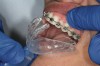
The technique for fabrication over orthodontic brackets is outlined in the accompanying figures. Although the two clear trays mentioned above in the previous non-orthodontic bleaching will work, the 1.5-mm thicker tray (1.5 Full Arch Boil & Form, Archtek, Inc) has the advantage of less shrinkage, which means it will cover more brackets and teeth (Figure 2). One difference in the insertion technique from a normal tray is that the tray should be inserted from a facial direction to avoid the wires and brackets causing the ends to fold (Figure 3). The water is heated until it almost boils, then the tray is waved in the hot water until the front edge begins to curl. If it continues too long in the water, it will shrink too much to fit over the brackets. If it touches itself, it will bond and be useless. Once the tray is softened, it is removed and the curled-in edges quickly spread back open to avoid hanging on the brackets. Any excess hot water is shaken from the tray and the tray is inserted from the facial direction. The patient’s lips must be relaxed to allow insertion of the softened tray. Once in the mouth, finger adaptation is used to form the tray over the brackets on the facial and the lingual (Figure 4). When this is completed, the patients close onto their posterior teeth and applies suction to form the tray with their lips (Figure 5). The tongue can also be used to push the tray against the lingual of the arch. When the tray has completely cooled in the mouth, the edges are disengaged from the brackets (Figure 6). The tray can then be removed, and the result is a custom-fitted tray made directly in the mouth over the braces (Figure 7). A pair of scissors can be used to remove any excess, as well as to remove the tray handle (Figure 8). The tray is reinserted to ensure that the occlusion is comfortable, and the tray handles have been removed smoothly (Figure 9). If needed, an acrylic trimming bur can be used to smooth where the handle was adapted. The mandibular tray can be fabricated in the same manner, although it is more difficult to fit. Only one tray is worn at a time, since the trays are constructed with the patient occluding into MI and are somewhat bulky. The best regime is to alternate nights of wear.
Bleaching Material for Caries Control
In conjunction with a custom-fitted tray made directly in the mouth over the orthodontic bracket is the use of an appropriate-viscosity carbamide peroxide material. Bleaching materials are ideal to use in the tray because their high viscosity maximizes contact time and minimizes leakage from the tray. Tray application is ideal overnight because the carbamide peroxide bleaching materials are effective for overnight application. If this is not reasonable, then the carbamide peroxide can be used for daytime use at a minimum of 2 hours. The one disadvantage of bleaching materials is the relative cost for long-term use. Typical orthodontic wear uses about one syringe for 3 to 4 nights when using a 10% carbamide peroxide product, and the refill kits of four syringes cost about $4 per syringe, so the additional cost for treatment over a 2-year treatment regime would be about $500. However, compared to the cost of restorative treatment and the cycle of replacement restorations that could be avoided, this may be minimal. Other options to be considered are existing OTC products, but none has the appropriate consistency to be as efficacious. Currently available OTC products (Glyoxide, GlaxoSmithKline Consumer Healthcare, www.gsk.com, and CVS Antiseptic Oral Cleanser, CVS Corp, www.cvs.com) are much more affordable but lack extensive amounts of carbopol thickening agent, thus are not maintained in the tray as long as dentist-provided bleaching agents. OTC products can be worn in the tray for a minimum of 1 hour, and still provide some additional cleaning. Whichever material is selected, only the amount that will cover the tooth surface without excessive leakage from the tray should be utilized to conserve materials. It is wise to have patients demonstrate use prior to dismissal from the office to ensure they understand the location and amount of material to use (Figure 10).
Carbamide Peroxide (CP) and its Antibacterial Properties
There are two basic formulations of peroxide materials used in tray bleaching. The initial tray ingredient in the original 1989 article was carbamide peroxide, which is active for 2 to 10 hours. Hydrogen peroxide has also been introduced, but is only active for up to 1 hour, so it is primarily for daytime use in bleaching. Ten percent CP is the commonly used percentage in tooth-bleaching procedures and is the most thoroughly researched CP formulation. It decomposes into 6.5% urea and 3.5% peroxide. The urea further breaks down to ammonia and carbon dioxide. Peroxide breaks down to water and oxygen. Carbopol (carboxy polymethylene polymer) is added to many commercial bleaching preparations because it increases the viscosity of the gel, increases contact time, and slows the release of oxygen from CP.11 Adding carbopol to CP preparations extends the maximal oxygen release time up to 10 hours, depending on how it is measured.12,13 The antibacterial properties of CP are well documented, as the original material was marketed as an oral antiseptic. In addition, artificially demineralized fissures (to simulate caries) inoculated with lactobacillus, and then treated with 10% CP gel for 2 hours showed no subsequent growth of lactobacillus when plated.14 The authors of this study concluded that 10% CP penetrated the carious fissures and killed the lactobacillus. It has also been shown that 10% CP inhibited growth of Streptococcus mutans and lactobacillus in vitro and reduced levels of salivary lactobacillus in vivo. The hydrogen peroxide products used in bleaching are not as effective for caries control since they do not contain urea.
Effect on Saliva, Plaque, Caries, and Gingival Health
Ammonia resulting from carbamide (urea) degradation plays a significant role in modifying salivary and plaque pH. In the 1960s, it was demonstrated that application of urea solutions to plaque resulted in an initial rapid rise in pH followed by a slow fall. The rise in plaque pH was related to urea concentration.15 More recently, 10% CP applied by wearing a custom tray resulted in a significantly increased salivary pH after 5 minutes of wear even though the CP products tested had an acidic pH (4.8 to 5.2). Salivary pH remained elevated above 8 for the 2 hours of tray wear for the test period.16 The buffering effect of CP in custom trays extends to plaque pH; measurements of plaque pH during 2 hours of CP application by custom tray showed that mean final plaque pH was significantly higher (8) than baseline (7).17 These results confirm the buffering effect of urea on saliva, since the normal urea concentration in saliva has a significant role in elevating plaque pH and in negating the rise in plaque pH after sugar challenge.18 The critical pH at which enamel and dentin begin to dissolve is 5.2 to 5.7 for enamel, and 6 to 6.5 for dentin.19 These studies demonstrate elevation of plaque and salivary pH significantly above these levels; this presumably results in a lower rate of caries.20 Elevation of saliva pH by CP also allays fears that acidic bleaching agents may cause enamel erosion. It is important to note that bleaching agents that contain hydrogen peroxide, but not CP, do not have these pH elevating effects, since it is the urea released from CP that causes elevation of plaque and salivary pH. Thus hydrogen peroxide-based agents would not necessarily have the same cariostatic benefits.
A similar study confirmed that salivary urea levels strongly correlated with plaque pH, very possibly causing a lower caries rate than controls or transplanted patients.21 This confirms the assumption that elevation of salivary and plaque pH by a constant source of salivary urea (for example from CP bleaching agents) may inhibit caries. Such caries inhibition has been demonstrated in the rat model, where topical application of 10% CP significantly reduced plaque accumulation and numbers of smooth surface enamel lesions.22
Side Effect of Bleaching for Caries Control
The technique for using bleaching materials for caries control has been previously reported in elderly patients.23 The rise in pH creates an environment in which caries cannot flourish. However, because it creates a basic pH, then calculus is more likely to form.24 It has been noted in the orthodontic patients that more calculus is present, often getting in the channels for the wires. However, it was determined that cleaning calculus was more reasonable than dealing with caries.
The other possible side effect is that the teeth may get whiter. However, for most patients in orthodontic treatment, this is desirable. For teenagers, this may be the motivating reason to wear the tray with the bleaching material, rather than the hope to avoid tooth decay.
Expressed Concerns
Concern is often expressed about the impact the bleaching material will have on the orthodontic bond strength. However, research has shown that the oxidation process of bleaching will actually strengthen the polymerization of the composite-bonded brackets by further curing the composite.25 Generally, composite cures only about 70%, so the addition of carbamide peroxide further increases the bond strength of the brackets. The opposite of this is true if bleaching is performed before bonding. In that case, the residual oxygen in the tooth reduces the bond strengths by 25%.26 Patients should wait at least 2 weeks after bleaching before any bonding procedure is attempted, to allow the complete dissipation of the oxygen from the enamel.27 However, once the bonding has been polymerized, then bleaching over the bonding will further poly¬merize the composite.
The second concern expressed of bleaching during orthodontic treatment is that there will be a “yellow spot” remaining after the bleaching. However, this has not been shown to be true either, as the peroxide passes easily through the tooth in 5 to 15 minutes,28 and will bleach under any composite or veneers29 already in the mouth (Figure 11 and Figure 12). If there were to be any yellow spots, those are most likely the residual composite from the bonding procedure, which will be embedded into the tooth at least 25 µm (Figure 13). Abrasion techniques must always be used after debonding orthodontic brackets to remove this composite. Even if there were a chance of a yellow spot, the simple solution would be to re-bleach the teeth. However, it has been shown that a tooth cannot be "spot bleached" due to the easy passage of peroxide from facial to lingual, and all clinical examples of bleaching during orthodontics have not shown any hint of an unbleached spot.
Concern has been expressed about the long-term use of the material, and the swallowing of material. However, the safety of 10% carbamide peroxide has been demonstrated pre-bleaching in use in newborn infants, and in previous long-term uses.30-32 The original product (Proxigel) was approved as Generally Recognized as Safe (GRAS) for use as an oral antiseptic by the US Food and Drug Administration for the life of the patient.33
Additionally, the long-term treatment of tetracycline patients has shown no detrimental effects on the teeth,34,35 and the 20-year history of research on the technique36,37 has shown the low-concentration, neutral-pH bleaching products from reputable manufacturers to be as safe to the teeth as normally ingested food stuffs and drinks. The more recent review of all the literature on safety by the European market further strengthens the safety of 10% carbamide peroxide.38
Additional Benefits of the Tray
In addition to having a custom-fitted tray that provides a carrier for the bleaching material to remove the plaque and elevate the pH, the tray also provides additional benefits. Because it was made with the patient occluding into maximum intercuspation, the patient has a stable MI bite registration in which to rest. Often during orthodontic therapy, there may be times when one tooth hits high, and becomes sore. The tray levels the occlusion so all teeth are in contact and provides a relief to occlusal trauma even when no bleaching material is added.
Additionally, because the tray covers the brackets and wires of the anterior portion of the mouth, it provides protection from the irritations to the lips and cheeks of orthodontic hardware, much in the same manner as wax, but much smoother. The oral antiseptic properties of the bleaching material also help with ulcer healing, because this was the original use of carbamide peroxide. The bleaching material also helps in controlling malodor, since it provides a bubbling action to clean the teeth of food debris, as well as provide a bacteriostatic cleaning of interproximal spaces from its oral antiseptic activity.
As has been noted earlier, the disadvantage of the tray options is that they only come in one size. Hence, the tray fabricated in this manner may not cover all the teeth (Figure 14). Because the tray was made with the patient occluding into MI, this does not create an occlusal problem. The question concerns whether the teeth not covered will be protected. However, because the elevation of the pH is the primary mechanism for reducing caries activity rather than plaque removal, it may not be as critical to cover all teeth, but rather have a tray that will hold the 10% carbamide peroxide in place during the night to elevate the pH above that which tooth decay can occur. When cross elastics are worn during orthodontic treatment, this technique cannot be used. Other options used during orthodontic therapy when elastics are being worn is to squirt the 10% carbamide peroxide material directly into spaces that are hard to clean for the mechanical debridement of those areas.
At this time, it is unknown whether this technique needs to be applied continually, or if it can be done for a week to clean, then do every other or third day. More research is needed in this area as to the elevation of the pH and how long it takes to drop below the critical levels to allow caries to progress, as well as the amount of plaque removed and how long takes it take to rebuild. This may vary from patient to patient. Disclosing tablets may show effectiveness over time. Additional cleaning appointments for the increased amount of calculus may need to be included in orthodontic plans.
As with any bleaching technique, sensitivity may be a side effect.39 However, to date, the sensitivity associated with orthodontic therapy exceeds any noted during this process. Additionally, the use of potassium nitrate in the bleaching materials, or the topical application of potassium nitrate, should help any problems.40,41 The use of orthodontic trays for both bleaching application and sensitivity application is another adjunct to orthodontic therapy.
Conclusion
A technique has been presented to fabricate a thermoplastic tray directly in the mouth over orthodontic brackets without removal of the brackets and without traditional impression techniques. The fabrication of this tray allows the patient to use 10% carbamide peroxide nightly as a means to reduce plaque and elevate the pH in the mouth above that which will cause tooth decay. The goal of this technique is to reduce or eliminate the need for restorations to restore white-spot and caries lesions after orthodontic treatment. No negative sequelae have been noted when this technique is used clinically, other than the additional cost of the trays and material.
Acknowledgments
Thanks to Dr. Michael Rogers, orthodontist in Augusta, Georgia, and Dr. Eladio DeLeon, Goldstein Chair of Orthodon¬tics for Medical College of Georgia, for their help in developing this technique.
Disclosure
Dr. Haywood has been a consultant to and/or received grant/product support from Glaxo SmithKline, Archtek, Inc, and OraTech, LLC, as well as worked with many bleaching companies.
References
1. Haywood VB. Tooth Whitening: Indications and Outcomes of Nightguard Vital Bleaching. 2007; Quintessence Publishing Company Inc: Hanover Park, Ill.
2. Bentley CD, Leonard R, Crawford JJ. Effect of whitening agents containing carbamide peroxide on cariogenic bacteria. J Esthet Dent. 2000;12(1):33-37.
3. Shapiro WB, Kaslick RS, Chasens Al, Eisenberg R. The influence of urea peroxide gel on plaque, calculus and chronic gingival inflammation. J Periodontol. 1973;44:636-639.
4. Reddy J, Salkin LM. The effect of a urea peroxide rinse on dental plaque and gingivitis. J Periodontol. 1976;47:607-610.
5. Zinner DD, Duany LF, Chilton NW. Controlled study of the clinical effectiveness of a new oxygen gel on plaque, oral debris and gingival inflammation. Pharmacol Ther Dent. 1970;1:7-15.
6. Shipman B, Cohen E, Kaslick RS. The effect of a urea peroxide gel on plaque deposits and gingival status. J Periodontol. 1971;42:283-285.
7. Napimoga MH, de Oliveira R, Reis AF, et al. In vitro antimicrobial activity of peroxide-based bleaching agents. Quintessence Int. 2007;38:329-333.
8. Gurgan S, Bolay S, Alacam R. Antibacterial activity of 10% carbamide peroxide bleaching agents. J Endod. 1996;22:356-357.
9. Haywood VB, Caughman WF, Frazier KB, Myers ML. Fabrication of immediate thermoplastic whitening trays. Contemporary Esthetics and Restorative Practice. 2001;5:84-86.
10. Oliver TL, Haywood VB. Efficacy of nightguard vital bleaching technique beyond the borders of a shortened tray. J Esthet Dent. 1999;11:95-102.
11. Haywood VB. Nightguard vital bleaching: a history and products update: part 1. Esthetic Dentistry Update. 1991;2(4):63-66.
12. Matis BA, Gaiao U, Blackman D, et al. In vivo degradation of bleaching gel used in whitening teeth. J Am Dent Assoc. 1999;130:227-235.
13. Matis BA. Degradation of gel in tray whitening. Compend Contin Educ Dent. 2000;21(28):S28-S35.
14. Amaechi BT, Barghi N, Jouett RM, Summit J. Bacteriocidal effects of carbamide peroxide bleaching gel [abstract 3245]. IADR/AADR/CADR 83rd General Session (March 9-12, 2005) Available at: http://iadr.confex.com/iadr/2005Balt/techprogram/abstract_58536.htm. Accessed December 1, 2009.
15. Kleinberg I. Effect of urea concentration on human plaque pH levels in situ. Arch Oral Biol. 1967;12:1475-1484.
16. Leonard RH Jr, Bentley CD, Haywood VB. Salivary pH changes during 10% carbamide peroxide bleaching. Quintessence Int. 1994;25:547-550.
17. Leonard RH, Austin SM, Haywood VB, Bentley CD. Change in pH of plaque and 10% carbamide peroxide solution during nightguard vital bleaching treatment. Quintessence Int. 1994;25(12):819-823.
18. Didbin GH, Dawes C. A mathematical model of the influence of salivary urea on the pH of fasted dental plaque and on the changes occurring during a cariogenic challenge. Caries Res. 1998;32:70-74.
19. Eliasson S, Krasse B, Soremark R. Root caries. A consensus conference statement. Swed Dent J. 1992;16:21-25.
20. Hoppenbrouwers PM, Driessens FM, Borggreven JM. The vulnerability of unexposed human dental roots to demineralization. J Dent Res. 1986;65(7):955-958.
21. Peterson S, Woodhead J, Crall J. Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res. 1985;19:796-799.
22. Firestone AR, Schmid R, Muhlemann HR. Effect of topical application of urea peroxide on caries incidence and plaque accumulation in rats. Caries Res. 1982;16:112-117.
23. Haywood VB. Bleaching and caries control in elderly patients. Aesthetic Dent Today. 2007;1:42-43.
24. Dawes C. Why does supragingival calculus form preferentially on the lingual surface of the 6 lower anterior teeth? J Can Dent Assoc. 2006;72:923-926.
25. Tanner JC, Smith BL, Rueggerberg FA, Haywood VB. Effect of dentist-prescribed home bleaching on orthodontic bracket retention. J Dent Res. 2001;80(#1359):205.
26. Lai SCN, Tay FR, Cheung GSP, Mak YF, Carvalho RM, Wei SHY, Toledano M, Osorio R, Pashley DH. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81(7):477-481.
27. Haywood VB. The “bottom line” on bleaching 2008. Inside Dentistry. 2008;4(2):82-89.
28. Cooper JS, Bokmeyer TJ, Bowles WH. Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod. 1992;18:315-317.
29. Haywood VB, Parker MH. Nightguard vital bleaching beneath existing porcelain veneers: a case report. Quintessence Int. 1999;30(11):743-747.
30. Munro IC, Williams GM, Heymann HO, Kroes R. Use of hydrogen peroxide-based tooth whitening products and its relationship to oral cancer. J Esthet Restor Dent. 2006;18(3):119-125.
31. Li Y. The safety of peroxide-containing at-home tooth whiteners. Compend Contin Educ Dent. 2003;24(4A):384-389.
32. Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int. 1992;23:471-488.
33. Haywood VB. The Food and Drug Administration and its influence on home bleaching. Current Opinion in Cosmetic Dentistry. 1993:12-18.
34. Matis BA, Wang Y, Eckert GJ, Cochran MA Jiang T. Extended bleaching of tetracycline-stained teeth: a 5-year study. Oper Dent. 2006;31(6):643-651.
35. Leonard RH Jr, Haywood VB, Caplan CJ, et al. Nightguard vital bleaching of tetracycline-stained teeth: 90 months post treatment. J Esthet Restor Dent. 2003;15(3):142-152.
36. Haywood VB. Considerations for vital nightguard tooth bleaching with 10% carbamide peroxide after nearly 20 years of proven use. Inside Dentistry. 2006;2(7):2-5.
37. Ritter AV, Leonard RH Jr, St Georges AJ, et al. Safety and stability of nightguard vital bleaching: 9 to 12 years post-treatment. J Esthet Restor Dent. 2002;14(5):275-285.
38. European Commission: Scientific Committee on Consumer Products: March 2005 SCCP/0844/04.
39. Swift EJ. Critical appraisal: at-home bleaching: pulpal effects and tooth sensitivity issues, Part 1. J Esthet Restor Dent. 2006;18(4):225-228.
40. Haywood VB, Caughman WF, Frazier KB, et al. Tray delivery of potassium nitrate-fluoride to reduce bleaching sensitivity. Quintessence Int. 2001;32:105-109.
41. Pashley DH, Tay FR, Haywood VB, et al. Consensus-based recommendations for the diagnosis and managements of dentin hypersensitivity. Inside Dentistry. 2008;4(9):Special Issue.
About the Author
Van B. Haywood, DMD Professor
Director of Dental Continuing Education
Department of Oral Rehabilitation
School of Dentistry
Medical College of Georgia
Augusta, Georgia