You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The lack of masticatory function is not the only untoward consequence of tooth loss. The physiologic modeling and remodeling of alveolar bone, resulting in lost horizontal and vertical ridge dimensions, is normally inevitable. Pietrokovski and Massler1 as well as Johnson2 proved this more than four decades ago. Animal studies performed by Araujo and Lindhe3 reported significant reduction of alveolar ridge dimension following extractions. Schropp et al4 demonstrated this phenomenon in humans, following 12 months of healing after removal of a single tooth. This bone loss can result in ill-fitting removable prostheses and difficulty in placing endosseous implants in their most favorable positions. The early phases of implant dentistry typically started with surgical assessments of the proposed implant sites, and fixture placement into the sites with adequate bony support for implant placement. Although osseointegration was often accomplished, functional, mechanical, and esthetic compromises were accepted.
As complications regarding prosthesis loosening, porcelain, abutment, abutment screw, and even implant fractures became more common, clinicians began to search for greater efficacy in implant therapy in an effort to eliminate the regularity of these problematic events. Restoring lost alveolar bone to facilitate biomechanically favorable implant placements was an eventual goal. Over the past two decades, numerous investigators have presented countless methods of accomplishing this goal. The purpose of this paper is to present a novel method of alveolar ridge augmentation that can lead to minimally invasive implant placement in regenerated sites.
Principles of Horizontal Space Maintenance
Bone has an astounding capacity for regeneration. Beyond the dense cortex found on bone’s outer surface, trabecular bone is often rich in undifferentiated mesenchymal stem cells. These cells, stimulated by the appropriate growth factors, have the ability to transform or differentiate into osteoblasts capable of bone formation. This treatment concept has resulted in successful regeneration of “space maintaining” defects, such as four-walled extraction sockets and well-contained periodontal lesions. These types of defects provide naturally occurring sites, where isolation of invading soft-tissue cells, via barrier membranes (guided bone regeneration [GBR], guided tissue regeneration [GTR]), can facilitate ingrowths of these osteoblastic cells and bone regeneration. This is not the case when bony walls are deficient or missing. Collapse of the membrane-protected space by the overlying flap is unavoidable in many situations, resulting in suboptimal regenerative outcomes.
One commonly utilized method of horizontal regeneration is the use of intraoral autogenous block grafts. The blocks are harvested from the patient’s symphisis or ramus. The donor sites are surgically isolated, and the appropriately sized blocks are “cut” with either rotary instruments, reciprocating saws, or piezo electric saws and removed. The perforation of cortical bone has been proven to enhance bone regeneration.5 The donor sites are then treated with bone replacement grafts and/or haemostatic agents, as advocated by Misch,6 and closed. The dense cortical bone at the recipient site is decorticated to facilitate vascularity in growth and migration of cells into the desired regenerated site. The block is then closely adapted to the recipient site with fixation screws. Tension-free closure of the overlying flap is achieved, and time to allow for incorporation of the block precedes fixation screw removal and implant placement. This technique is widely used and investigators report varying levels of success.
One of several drawbacks to this technique is resorption of the block, resulting in suboptimal bone regeneration. Cordaro7 et al reported approximately 25% horizontal graft reduction using this technique. Methods to reduce the resorption of autogenous block grafts have been tested, often with positive results. Von Arx and Buser8 evaluated the efficacy of combining block grafts with anorganic bone particulate and resorbable porcine collagen membranes. They reported minimal “surface resorption” with this technique. In the rabbit model, Kim and co-workers9 demonstrated the synergistic effect of collagen membranes and block grafts, and further demonstrated that the application of a “double membrane” technique resulted in smaller amounts of resorption compared to a single-layer technique.
The resorption of block grafts, the expense associated with auxiliary materials necessary to minimize resorption, and the morbidity associated with donor sites leads clinicians to search for alternative methods for ridge augmentation. One commonly used technique is the application of a rigid mesh to maintain regenerative space. This is not a new principle. Boyne10 presented a technique of combining autogenous bone, harvested from the iliac crest, and protecting it from resorption by a titanium mesh. He demonstrated minimal resorption after 3 to 10 years in the edentulous maxilla. In the canine model, Thoma and co-workers11 evaluated several methods of ridge augmentation of experimental defects when grafting with recombinant human bone morphogenetic protein-2 (rhBMP-2) combined with bone graft materials or titanium mesh. They found significant differences between modalities. Demineralized grafts proved inadequate for space maintenance, resulting in collapse of the grafted sites and inadequate ridge dimensions for implant placement. When rhBMP-2 was protected by a titanium mesh, or combined with mineralized, slowly resorbing bone graft, more successful regeneration was reported. Von Arx and Kurt12 presented a case series of implants placed with fenestration or dehiscence defect, which were grafted with autogenous bone and titanium mesh. They reported an average of 93.5% defect resolution. The protective mechanism of rigid mesh is not fully understood, however it has been demonstrated to enhance even autogenous block grafting procedures. Rocuzzo et al13 demonstrated less graft resorption when block grafts were “covered” with titanium mesh. These studies demonstrate the efficacy of a rigid mesh in alveolar ridge augmentation.
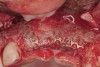
Disadvantages to this technique include time necessary for mesh shaping, manipulation, and fixation. But perhaps the greatest shortcoming of titanium mesh is the necessity of its removal. This requires wide flap reflection and frequently sharp dissection (Figure 1). Often, a dense, soft-tissue layer is found over the mesh, referred to by Boyne10 as a “pseudo-periosteum.” This tenacious soft tissue is tightly bound to the mesh and underlying bone, requiring sharp dissection and elevation of the material. This adds significant operative time to the procedure. This type of soft tissue is shown in Figure 2; the mesh removed at the time of implant placement still has the tissue attached following sharp dissection and elevation. The wide flap reflection and time needed to remove the mesh increases morbidity associated with the second surgical procedure, primarily intended for implant placement. The evolution of this technique has led to the development of a rigid but resorbable mesh. The challenge for clinicians and researchers is to find a resorbable mesh capable of space maintenance comparable to titanium mesh, yet composed of a material that is biocompatible and does not compromise regenerative outcomes.
Buchmann et al14 demonstrated mild inflammatory reactions to synthetic guided tissue regeneration (GTR) barriers in humans. When comparing membranes composed of polylactic acid (PLA) and glycolide-lactic-co-polymer (PGL) in mandibular furcation lesions, both resulted in vertical attachment gain. The enzymatic release from polymorphonuclear leukocytes (PMNs) was slightly more prolonged for the PLA barriers, but there was no clinical difference noted. The use of synthetic polymers serving as tissue-exclusionary barriers in periodontal therapy is time-tested and clinically proven. Karapataki et al15 found that intrabony periodontal defects treated with resorbable PLA membranes yielded comparable results compared to non-resorbable (ePTFE) barriers. In the animal model, Laurell et al16 reported indistinguishable comparisons of native periodontal tissues and those regenerated with PLA barrier utilized in GTR procedures.
One of these materials approved for clinical use is a mesh composed of a copolymer of 85% polylactide and 15% polyglycolide. This material is resorbed in vivo over approximately 12 months time and reportedly maintains its structural integrity for about 2 months, prior to its more rapid breakdown into lactic and glycolic acids before it is eliminated via hydrolysis in the form of water and carbon dioxide (CO2). The biocompatibility of this material defends against untoward patient reactions and wound complications, and it is designed to protect space for bone regeneration. Either bone particulate graft or recombinant bone stimulatory proteins can be placed between the alveolar ridge and the resorbable mesh. Lane and co-workers17 demonstrated that when a poly-lactic-co-glycolic acid (PLGA) carrier is combined with rhBMP-2 in the animal model, no adverse local or systemic effects were observed. They also reported clinical efficacy of this combination in bone regeneration of surgically created bony defects without any ectopic bone formation. Owen and co-workers18 investigated varieties of PLGA membranes and found the potential for sustained drug release in treating periodontal defects.
Clinical Applications
Case One
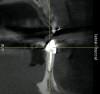
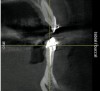
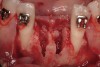
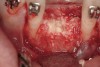
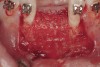
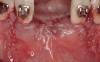
The patient, a 39-year-old woman, presented at the inception of multidisciplinary therapy. Prior to starting comprehensive orthodontic treatment, it was determined that tooth Nos. 24 and 25 were deemed hopeless (Figure 3). Cross-sectional views obtained from cone-beam computer tomography (CBCT) demonstrated deficient labial and lingual cortices at the time of presentation (Figure 4 and Figure 5). Full-thickness flap reflection and delicate extractions were done, utilizing periotomes and forceps and taking precaution to avoid unnecessary trauma and further bone loss; tooth Nos. 24 and 25 were removed (Figure 6). Following thorough debridement with ultrasonic and hand instrumentation, the defects were obturated with a mineralized allograft (freeze-dried bone allograft [FDBA], Musculoskeletal Transplant Foundation [MTF] Tissue Bank, www.mtf.org) (Figure 7). For purposes of graft containment, a traditional barrier membrane may have been chosen; however, due to the compression of the overlying flap and an anticipated 6 to 9 months before implant placement was expected to occur, a thin or knife-edge crest ridge anatomy could result in this area. Therefore, a rigid, resorbable mesh was utilized to provide graft containment and space maintenance (Figure 8). This PLGA-composed mesh was trimmed extraorally to the desired size of the defect, then placed in a sterile, warm water bath at 70°C for about 10 seconds. This temporary warming allows for 3-dimensional (3-D) contouring of the flat mesh to the desired shape needed to reconstruct the alveolar ridge. To prevent movement of the mesh in situ, it was affixed to the labial cortex with screws composed of the same PLGA polymer. A dermal allograft was placed over the graft site to augment the volume of soft tissue in this area of typically thin keratinized mucosa (Figure 9). The flaps were closed in a tension-free manner after a facial periosteal-releasing incision (Figure 10).
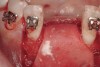
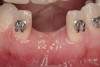
Active tooth movement was complete approximately 6 months after surgery, and a second CBCT scan was taken to evaluate the site prior to implant-placement surgery. With the adequate presence of both hard and soft tissues in the edentulous area (Figure 11), it was determined that no further tissue augmentation was required to place implants in their prosthetically desired positions. At this point, the virtue of not having to deal with the removal of the mesh was apparent. Because regeneration was successful, and surgical re-entry to remove a titanium mesh and fixation screws was unnecessary, a flapless, computer-guided surgery could be considered utilizing software to fabricate the templates (Figure 12). This would reduce morbidity for the patient and enhance implant placement accuracy. Because the procedure was minimally invasive, postoperative bleeding was minimal, making it easier for the orthodontist to replace the arch wire and attached denture teeth for fixed provisionalization postoperatively.
Case Two
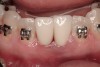
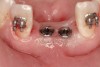
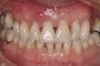
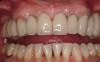
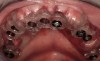
A 46-year-old woman presented with advanced chronic periodontitis and had already lost several maxillary and mandibular teeth (Figure 13 and Figure 14). She initially complained of pain in the maxillary anterior sextant, where a draining sinus tract between tooth Nos. 7 and 8 was evident. The patient desired a fixed restoration and was unwilling to wear a removable prosthesis, even on a temporary basis, if possible. It was decided that tooth Nos. 2, 6, 11, and 15 would be retained to support a fixed, provisional bridge, while the patient’s remaining teeth would be extracted and bone augmentation performed, followed by implant placement (Figure 15). Extraction of the maxillary teeth with the exception of these four teeth was done to simplify provisionalization.
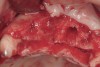
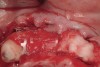
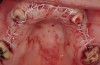
The patient returned to the surgeon for bilateral maxillary sinus grafting and anterior ridge augmentation. Because the patient was a heavy smoker (more than 1.5 packs per day) and due to the magnitude of the desired amount of bone regeneration, a biologic mediator or growth factor was chosen to enhance augmentation and compensate for a suspected compromise in systemic wound healing caused by her substantial amount of smoking. This is an empirical statement regarding the efficacy of the use of growth factors for these purposes. Following flap reflection, bilateral sinus grafts were performed, utilizing a lateral approach with piezo surgery for osteotomy preparation; both sinuses were grafted with rhBMP-2/absorbable collagen sponge (ACS). The residual extraction sites were also obturated with this grafting material. The area of tooth Nos. 7 and 8 presented a unique challenge, because the facial wall of the extraction sockets was totally missing (Figure 16). This graft material, though osteoinductive, lacks any space-maintaining properties. Therefore, a resorbable mesh was used to protect the 3-D regenerative space desired for proper implant placement (Figure 17). The flaps were then closed with a monofilament suture and the provisional bridge was recemented (Figure 18).
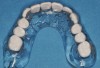
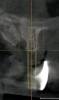
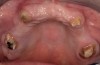
The patient returned approximately 5 months after the augmentation procedure for implant placement. Prior to this step, a CT scan was taken with the patient wearing a radiopaque scanning appliance, which was based on the final prosthetic treatment plan (Figure 19 and Figure 20). Planning software demonstrated adequate 3-D volume for implant placement in the regenerated area where rhBMP-2/ACS and PLGA mesh were combined. Removal of the provisional bridge permitted visualization of a healthy and symmetrical edentulous ridge (Figure 21). A tooth- and soft-tissue-supported template was seated to facilitate flapless, computer-guided implant placement (Figure 22). Because the patient desired a fixed, final prosthesis and a “full complement” of teeth with the exception of third molars, nine implants were placed. Although a full-arch prosthesis may be supported with fewer implants, the treatment plan anticipated that one or more implants could fail to osseointegrate due to the patient’s heavy smoking habit. With nine implants placed initially, both the surgeon and restorative dentist decided that a full-arch prosthesis would still be possible despite the potential loss of one or two implants. It was noteworthy that in the maxillary anterior sextant, the resorbable mesh in the area of tooth Nos. 7 and 8 enabled adequate hard- and soft-tissue dimensions for implant placements. Moreover, due to the resorbable properties of the mesh, a flapless, guided placement could be performed (Figure 23).
Discussion
Contemporary strategies for regenerating lost alveolar bone are frequently based on the concepts of “tissue engineering.” Bruder and Fox19 described the three “basic biologic elements” required for skeletal tissue regeneration: cells, growth and differentiation factors, and extracellular matrix scaffolds. As mentioned and demonstrated in this paper, host trabecular bone in healthy individuals is capable of providing the cellular population necessary for differentiation stimulated by the appropriate growth factors. The challenge for surgeons is to provide a matrix capable of supporting “cellular attachment, migration, and proliferation” as described by Bruder.19 The ideal graft material for bone augmentation is yet to be discovered. Many materials are superior to others in one or more aspects yet are inferior regarding other properties.
Autogenous and allogeneic block grafts are excellent in terms of providing 3-D space. Investigators such as Misch20 have reported excellent results concerning improved bone density at proposed implant sites; however, they did mention limitations of donor bone volume and potential for nerve damage to lower anterior teeth. Autogenous block grafts are also associated with significant resorption and donor site morbidity. Particulate grafts, which can be autogenous, allogeneic, xenogenic, or alloplasts, are easier to apply, though graft migration during and following surgery can be a challenge. Depending on their source and processing, they may be considered osteogenic, osteoinductive, osteoconductive, or a combination of these properties. Although autogenous bone can provide viable bone-forming cells, the limiting factor for its use is the amount that can be harvested for the appropriate defect being grafted. This type of grafting will always have varying degrees of donor site morbidity associated with it. Allografts have been utilized for decades in periodontics and oral surgery applications. These grafts are primarily osteoconductive, meaning they serve as a scaffold for passive cellular repopulation and substitution. In naturally occurring space-maintaining defects, this type of material is very successful. The demineralization of bone allograft has the potential for osteoinductivity, meaning that the acid-demineralization process exposes mineral-bound growth factors, such as bone morphogenetic proteins (BMPs). The interaction of these proteins with undifferentiated mesenchymal cells can influence these cells towards their differentiation down the osteoblastic pathway. Sato and Urist21 demonstrated this phenomenon of differentiation in an animal model.
One of the disadvantages of using demineralized bone for alveolar bone augmentation is its rapid resorption rate. Often this results in less than ideal 3-D regeneration of the alveolar ridge. Xenografts and alloplasts are exclusively osteoconductive bone grafts, with varying degradation rates. Some are even considered to be nonresorbable. These materials may provide better space maintenance compared to demineralized bone, yet their substitution with regenerated autogenous bone is questionable. This may limit the eventual bone-to-implant contact, or osseointegration necessary for long-term implant loading success. In a short-term study in humans, this theory, based on implant survival, has been refuted by Crespi et al.22 In the animal model, Araujo et al3 in their histologic analysis of extraction sockets speculated that a bone alloplast may have “retarded bone formation.” The regeneration of native bone by rhBMP-2, capable of sustaining functional loading, has been demonstrated in the animal model by Jovanovic et al.23 In a human multi-center study, Fiorellini et al24 demonstrated significant ridge dimensional preservation and gains when a therapeutic dose of rhBMP-2 delivered on an absorbable collagen sponge was used in anterior extraction sites with buccal wall deficiencies. Although a different application of rhBMP-2/ACS was utilized in the sinus graft, multicenter studies25,26 reported favorably regarding 2- to 3-year functional loading of implants placed in sites grafted with this material. A regenerated site without residual graft material may constitute the ideal scenario for ridge augmentation procedures. Bone xenografts and alloplasts also may require graft migration and containment similar to that of autogenous and allogeneic particulates.
With functional and esthetic demands becoming greater for implant therapy, it has never been more crucial to place implants into the most naturally occurring tooth locations as possible. This challenge is great when the alveolar ridge is damaged or severely resorbed. Clinicians desire to place implants into sites with adequate hard and soft tissues, where native bone underlying healthy keratinized mucosa provides the best possible scenario for success. A “moldable” mesh can provide this type of regenerative space. Whether passive graft materials or osteoinductive proteins are placed beneath the mesh, the desired outcome is the regeneration of a healthy ridge composed of native bone. As demonstrated in this article, the utilization of a resorbable mesh has one main advantage over titanium mesh: its removal is unnecessary. Not only does this simplify implant placement surgery, but it may also, under the appropriate circumstances, facilitate flapless, computer-guided implant surgery.
The cases selected in this article are representative of a larger number of patients treated in a private periodontal practice. Based on the severity of tissue destruction and the quantity of desired regeneration, variations in grafting materials were utilized. For this reason, a consistent treatment protocol was not presented. In areas where the number of osseous walls necessary for partial graft containment and sources of vascularity are present, osteoconductive therapy can be sufficient to achieve the desired result. When the goal of substantial regeneration may be considered ambitious due to extensive bone loss, a more active, osteoinductive solution can be pursued. The utilization of stimulatory proteins, such as rhBMP-2, can provide a means of “compensating” for the compromised regenerative potential of severe osseous defects.
The purpose of this article is to provide the rationale for utilizing novel, bioresorbable space-maintenance materials, combined with various osteoconductive and/or osteoinductive graft materials, in the attempt to regenerate lost alveolar bone for the purpose of placing endosseous dental implants. The technique presented is meant to serve as an alternative to older methods of grafting, such as the use of autogenous block grafts and titanium mesh techniques. The benefit of this newer technique is to avoid or minimize some of the morbidity and complications frequently associated with these other techniques.
Conclusion
As technology advances, sometimes at unnerving rates, patients will come to expect tooth replacement to mimic their natural dentition in all aspects, achieved with a process that is less and less invasive and time-consuming. It has been demonstrated that by eliminating the removal of a space-maintaining mesh combined with stimulatory rhBMP-2/ACS, ambitious bone regeneration and minimally invasive implant placement can be performed in a relatively timely manner with reduced morbidity for the patient.
References
1. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.
2. Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust Dent J. 1969;14(4):241-244.
3. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212-218.
4. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
5. Nishimura I, Shimizu Y, Ooya K. Effects of cortical bone perforation on experimental guided bone regeneration. Clin Oral Implants Res. 2004;15(3):293-300.
6. Misch CM, Misch CE. Autogenous mandibular bone grafts for reconstruction of ridge deficiencies prior to implant placement [abstract]. Int J Oral Maxillofac Implants. 1993;(8):117.
7. Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002;13(1):103-111.
8. von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17(4):359-366.
9. Kim SH, Kim DY, Kim KH, et al. The efficacy of a double-layer collagen membrane technique for overlaying block grafts in a rabbit calvarium model. Clin Oral Implants Res. 2009;20(10):1124-1132.
10. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87-91.
11. Thoma DS, Jones A, Yamashita M, et al. Ridge augmentation using recombinant bone morphogenetic protein-2 techniques: an experimental study in the canine. J Periodontol. 2010;81(12):1829-1838.
12. von Arx T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10(1):24-33.
13. Rocuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18(3):286-294.
14. Buchmann R, Hasilik A, Heinecke A, Lange DE. PMN responses following use of 2 biodegradable GTR membranes. J Clin Periodontol. 2001;28(11):1050-1057.
15. Karapataki S, Hugoson A, Falk H, et al. Healing following GTR treatment of intrabony defects distal to mandibular 2nd molars using resorbable and non-resorbable barriers. J Clin Periodontol. 2000;27(5):333-340.
16. Laurell L, Bose M, Graziani F, et al. The structure of periodontal tissues formed following guided tissue regeneration therapy of intra-bony defects in the monkey. J Clin Periodontol. 2006;33(8):596-603.
17. Lane JM, Yasko AW, Tomin E, et al. Bone marrow and recombinant human bone morphogenetic protein-2 in osseous repair. Clin Orthop Relat Res. 1999;(361):216-227.
18. Owen GR, Jackson JK, Chehroudi B, et al. An in vitro study of plasticized poly(lactic-co-glycolic acid) films as possible guided tissue regeneration membranes: material properties and drug release kinetics. J Biomed Mater Res A. 2010;95(3):857-869.
19. Bruder SP, Fox BS. Tissue engineering of bone. Cell based strategies. Clin Orthop Relat Res. 1999;(367 suppl):S68-S83.
20. Misch CM, Misch CE. The repair of localized severe ridge defects for implant placement using mandibular bone grafts. Implant Dent. 1995;4(4):261-267.
21. Sato K, Urist MR. Induced regeneration of calvaria by bone morphogenetic protein (BMP) in dogs. Clin Orthop Relat Res. 1985;197:301-311.
22. Crespi R, Capparè P, Gherlone E. Dental implants placed in extraction sites grafted with different bone substitutes: radiographic evaluation at 24 months. J Periodontol. 2009;80(10):1616-1621.
23. Jovanovic SA, Hunt DR, Bernard GW, et al. Long-term functional loading of dental implants in rhBMP-2 induced bone. A histologic study in the canine ridge augmentation model. Clin Oral Implants Res. 2003;14(6):793-803.
24. Fiorellini JP, Howell TeH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76(4):605-613.
25. Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63(12):1693-1707.
26. Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67(9):1947-1960
About the Author
Barry P. Levin, DMD
Clinical Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania
Private Practice
Periodontology and Dental Implant Surgery
Elkins Park, Pennsylvania
Fellow
International Team for Implantology (ITI)