You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Head and neck squamous cell carcinoma (HNSCC) is the eighth most common cancer worldwide with approximately 650,000 new cases reported annually.1 This statistic does not include various human malignancies that are not of epithelial origin (ie, carcinoma) such as sarcomas (eg, rhabdomyosarcoma, fibrosarcoma, neurosarcoma, etc), lymphomas, leukemias, skin adnexa malignant tumors, and melanomas. Furthermore, the above cited incidence rate is for primary neoplasms (excluding the jaws) and does not include metastases to the head and neck region from distant anatomical sites. In the United States, during 2007 there were 45,769 squamous cell carcinoma cases of the oral cavity and oropharynx, ranking it eighth in incidence among men and 14th among woman.2
Unfortunately and confusingly, in both peer-reviewed and non-peer-reviewed medical and dental journals in the English language, there are numerous published articles and advertisements that interchangeably use a variety of head and neck cancer terms to cite statistics, facts, and current research findings about "oral cancer" (Table 1). Closer scrutiny reveals that the authors are often reporting not only "oral cavity proper" carcinoma facts and statistics but often also those of oropharyngeal carcinoma, and, less often, hypopharyngeal (eg, laryngeal carcinoma), nasopharyngeal (eg, nasopharyngeal carcinoma), as well as non-epithelial malignancies (ie, sarcomas). Additionally, some reports include morbidity and mortality data from the nasal passages, paranasal sinuses, major salivary glands, and thyroid gland.
Of the various types of HNSCC, this article will solely deal with oral cavity squamous cell carcinoma (OCSCC) and oropharyngeal squamous cell carcinoma (OPSCC), which together comprise approximately 95% of head and neck cancers. Other carcinomas that can occur in the oral cavity and oropharynx but will not be discussed include minor salivary gland adenocarcinoma and lymphoepithelioma.
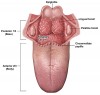
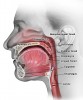
Anatomically, the oral cavity extends from the skin-vermilion junctions of the anterior lips to the junction of the hard and soft palates above, to the line of circumvallate papillae below, and is divided into the following specific areas: lip, anterior two-thirds of tongue, buccal mucosa, floor of mouth, maxillary and mandibular gingiva, retromolar trigone, and hard palate. The oropharynx is located between the soft palate superiorly and the hyoid bone inferiorly, and is continuous with the oral cavity anteriorly, and communicates with the nasopharynx superiorly and the supraglottic larynx and hypopharynx inferiorly.3 The oropharynx is divided into the following sites: base of tongue, which includes the pharyngoepiglottic folds and the glossoepiglottic folds; tonsillar region, which includes the fossa and the anterior and posterior pillars; soft palate, which includes the uvula; and the posterior and lateral pharyngeal walls4 (Figure 1 and Figure 2).
Human Papillomaviruses (HPV)
The human papillomaviruses (HPV) are a group of more than 130 unique types of small, double-stranded circular DNA viruses that can produce epithelial tumors of the skin and mucous membranes. Humans are their only known hosts and, interestingly, HPV is normally present in a small percent of the population. HPV lesions are thought to arise from the proliferation of infected basal keratinocytes. The infection typically occurs when epithelial basal cells of the host are exposed to the infectious virus through a disturbed epithelial barrier as would occur during sexual behaviors or following minor skin/mucosal abrasions. Once the epithelial cell is infected the viral multiplication is confined to the nucleus. HPV oncoproteins E6 and E7 are believed to be involved in the subsequent genetic alterations by interacting with tumor suppressor genes p53 and pRb, respectively.5
Some types of HPV cause the formation of common benign cutaneous warts (eg, verruca vulgaris), especially on the hands and feet, and do not spread easily; however, more than 40 types are sexually transmitted and spread very easily through genital contact. Of the sexually transmitted HPV types, some do not appear to cause cancer and are referred to as low-risk HPVs. Two examples of low-risk HPVs are HPV-6 and -11, which cause condyloma acuminatum (ie, genital warts) to appear on or around the genitalia or anus. Genital warts may appear within several weeks after sexual contact with a person who is infected with HPV or they may take months or years to appear, or they may never appear. On the other hand, approximately 15 types of sexually transmitted HPVs cause uterine cervical cancer and other types of cancer. These are called high-risk (oncogenic) HPVs and most frequently include types HPV-16 and HPV-18, which are responsible for 70% of cervical carcinoma.6 The genomes of high-risk HPV types 16 and 18 integrate into the host cells' DNA, which is considered the hallmark of malignant transformation. HPV infections also cause some carcinomas of the anus, vulva, vagina, penis, and, proven most recently, the oropharynx.5 It is the latter site that has gained so much notoriety within both the scientific community and lay press in the past few years. However, media has wrongly lumped high-risk HPV-associated carcinomas of the oropharynx together with squamous cell carcinomas of the oral cavity, which are caused by traditional social risk factors such as smoke tobacco and alcohol abuse, thereby creating confusion among dental clinicians.
It is important to note that 90% of genital HPV infections clear on their own within 2 years and that over the span of 7 to 10 years only 5% of persistent genital HPV infections will cause dysplastic changes of the uterine cervix that will ultimately result in squamous cell carcinoma (12,000 cervical carcinoma cases occur annually in the United States).7 Various risks factors such as smoking and numerous pregnancies may increase the risk of cervical cancer development in women persistently infected with high-risk HPV types. Although any person who is sexually active is at risk for sexually transmitted HPV infections, being in a long-term monogamous relationship with an uninfected partner minimizes the risk of genital HPV infection. Barrier techniques such as condom use have been associated with a lower rate of cervical cancer.6 Additionally, 5 years ago Gardasil® (Merk and Co., Inc.), a recombinant human papillomavirus quadrivalent vaccine (types 6, 11, 16, and 18), and two years ago Cervarix® (GlaxoSmithKline Biological), a recombinant human papillomavirus bivalent (types 16 and 18), were approved by the US Food and Drug Administration for use in women between the ages of 9 and 26 and 10 and 25, respectively. These HPV vaccines have been shown to be highly effective in preventing persistent infections with high-risk HPV types 16 and 18; Gardasil has also shown to be effective against genital warts.
OPSCC and OCSCC
For many years, researchers and clinicians believed OPSCC and OCSCC were homogenous entities with similar biological activity, etiopathogenesis, risk factors, clinical appearance, signs and symptoms, histopathology, treatment, and prognosis. The prototypical patient was an elderly (> age 50) man who had smoked cigarettes (or other forms of tobacco) and/or abused beverage alcohol for many years. The carcinogens within the tobacco products and the acetlyaldehyde component of the alcohol were known to be the primary components that resulted in multiple "hits" of DNA damage and loss of control of cell cycle events such as cellular reproduction and apoptosis (ie, programmed cell death).8 Many investigators reported a synergistic effect when these two chief etiologic factors were used simultaneously, and since the early 1950s it has also been shown that an individual's genetic makeup could contribute to a mucosal milieu of susceptibility (ie, field cancerization theory).8
Other etiologic factors of homogeneous OPSCC and OCSCC had also been emphasized, including age, gender, race, familial history, diet and nutrition (ie, lack of adequate fruits and vegetables), immunosuppressant diseases and therapeutic medications, and other lifestyle habits such as betel quid and maté consumption.9 Other risk factors such as viral infections, poor oral hygiene, poor dentition, periodontal disease, cannabis use, khat chewing, HIV infection, and alcohol content in mouthwashes had more inconsistent or limited evidence.9
Based on the prototypic profile of OCSCC/OPSCC and its related factors, it was optimistically thought that behavior modification strategies (eg, nicotine reduction methods and alcohol temperance) would lead to prevention of OCSCC/OPSCC, and, indeed, since the early 1980s the overall incidence of OCSCC has decreased approximately in parallel with the downward trend in smoking. In fact, the incidence of all age/gender categories of OCSCC has been declining since then except for the anterior two-thirds of the tongue in younger (ie, less than 45 years old) white women with no history of smoking or alcohol use.10 The specific reason for an increase in this cohort is unknown, since, in fact, only very rarely has another suspected risk factor, HPV-16 infection, been linked to OCSCC (approximately ≤ 2% to 3%).
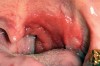
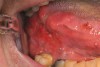
Clinically, it was thought that early prototypical OPSCC and OCSCC had identical potentially malignant (premalignant) appearing lesions—leukoplakia, erythroplakia, or erythroleukoplakia (Figure 3). Studies have shown that the erythroplakic component is most likely to microscopically demonstrate significant amounts of epithelial dysplasia.8 Painless, ulcerative lesions then develop from the potentially malignant lesions and slowly enlarge until additional signs and symptoms appear (Figure 4), and eventually, if the lesion remains undiagnosed, locoregional cervical lymphadenopathy becomes apparent. Unfortunately, researchers have recently discovered that clinical, normal-appearing mucosa of the oral cavity and oropharynx can already harbor microscopic and molecular precancerous changes.1 Furthermore, a defined, observable clinical precursor lesion associated with oropharyngeal squamous cell carcinoma caused by HPV infection is not yet known.
Heterogeneous Sites
Within the past decade, epidemiological and molecular studies have revealed that, in fact, these two sites of squamous cell carcinoma are heterogeneous.11-15 To date, the most important revelation is that a unique subset of oropharyngeal carcinoma exists, caused by sexual transmission of certain types of HPV, especially type 16.16 The molecular evidence for HPV-16 infection in OPSCC is as strong as for cervical carcinoma with respect to high-risk DNA present, tumor specificity, E6/E7 expression, clonality-copy number, clonality-integration, and, to a lesser degree, clonality-variant analysis and malignant phenotype. HPV-associated OPSCC has also been shown to be a distinct molecular-genetic entity with respect to amount of wild versus mutant p53 and prRb, p16, cyclin D, epithelial growth factor receptor (EGFR), and survivin17-21 (Table 2). Furthermore, the gene expression profile for HPV-positive OPSCC has 89 genes with higher expression and two genes with lower expression.22 The HPV type distribution in HPV-positive OPSCC is 92% for type 16 with types 18, 33, 35, 45, and 59 each contributing a minor amount.23 HPV-16 seropositive individuals are at a distinct increased risk for oropharyngeal carcinoma compared to the oral cavity and other sites such as the larynx, nasal cavity, paranasal sinuses, and lips.24
Non-HPV-associated OPSCC have similar potentially malignant clinical changes as OCSCC. However, in addition, it has been shown that small lesions of HPV-associated OPSCC (stages T1 and T2), including some as small as several millimeters in diameter, may occur within the crypts of tonsillar and base of tongue but already have a significant cervical lymph node involvement (levels II and III) (Figure 5). The metastatic sites within the lymph nodes are cystic rather than solid, as seen in HPV-negative head and neck squamous cell carcinomas, and the primary tumor is typically unknown. HPV-positive OPSCC are consistently unassociated with dysplasias of the surface epithelium, exhibit lobular growth, and are permeated by infiltrating lymphocytes. Thus, it is more important than ever that dental healthcare providers perform careful and extensive extraoral head and neck examinations of all cervical lymph nodes. An additional warning symptom particularly important for detection of HPV-associated OPSCC includes referred pain to an ipsilateral ear that upon clinical examination lacks primary pathology.4
The discovery of heterogeneous epidemiologic and molecular study findings had dealt a serious and disquieting blow to the optimism of winning the battle against HNSCC through behavior modification. It now appears that an increasing epidemic of HPV-associated carcinoma is present, specifically OPSCC. In the United States, Northern Europe, and Australia, reports of increasing prevalence of oropharyngeal carcinoma due to HPV have ranged from 25% to 90%; currently, in the United States, OPSCC that is associated with HPV is thought to be approaching 50%.14,15,25 It must be emphasized that these studies have only shown a strong HPV risk factor association with squamous cell carcinoma of the oropharynx, not the oral cavity.
The increased incidence of OPSCC is thought to be due to a combination of nontraditional behavioral and environmental factors. Oropharyngeal squamous cell carcinoma patients' traditional risk factors have been supplanted by HPV infections related to sexual behavior changes such as increased number of sexual partners, number of oral-sex partners, history of sexually transmitted infections, inconsistent use of barriers, and young age at first intercourse. There does not seem to be a correlation with the number of oral-oral partners and young age at first oral sex.13,22-28 Thus, oral anogenital contact is an important route for the transmission of HPV to the oropharynx. In addition, marijuana use has been recently identified as a co-factor dependent on the intensity, duration, and cumulative years of smoking.14 It is speculated that the marijuana smoke may be more relevant than the substance's DNA-damaging carcinogens due the smoke's immunomodulatory effects.
Clinically, the HPV-positive head and neck squamous cell carcinomas are seen in higher socio-economic, younger (40 to 59 years old), white men (3.1:1 male/female ratio), with the chief risk factor being sexual behavior rather than tobacco/alcohol abuse (Table 2). Although HPV-negative tumors are seen at all oral cavity sites and the oropharynx, the HPV-positive tumors are confined most often to the tonsillar region (palatine tonsil) and the base of the tongue (lingual tonsil) and, to a lesser degree, the posterior/lateral wall of the pharynx, larynx, and paranasal sinuses. Researchers hypothesize that the base of the tonsillar tissue crypts act as traps for antigens as part of Waldeyer's ring of lymphoid tissue and that microtrauma occurs in their basal cell keratinocytes.29 The latter cells have been shown to be the precise site of HPV cellular invitation and DNA nuclear integration.29 Furthermore, this area of the oropharynx is an embryogenic transformation zone similar to the uterine's cervix transformation zone. Investigators do not know or understand why oropharyngeal HPV-16 infection is more prevalent in men, but it could be due to rates of infection in men versus women, patterns of sexual behaviors, or biological differences. It is also unknown how many oropharyngeal HPV infections persist and how many spontaneously clear in a similar manner to the cervix. It should also be noted that most surgical tonsillectomies do not remove 100% of palatine tonsillar tissue, and the lingual tonsil is not removed.
Investigators have also reported the heterogeneous histopathology of OPSCC and OCSCC.29 The HPV-negative tumors are usually keratinized to a variable degree, whereas the HPV-positive OPSCC are non-keratinized ("basaloid") although not undifferentiated since the tumor cells microscopically closely resemble the appearance of the reticulated epithelium—the specialized epithelium lining the tonsillar crypts.
Investigators have demonstrated that compared to HPV-negative tumors, HPV-positive carcinoma incidence is increasing. The Centers for Disease Control and Prevention (CDC) estimate of the annual burden of HPV-associated cancers in the US pre-vaccine (1998-2003) from population-based cancer registries, stratified by anatomic subsites and the incidence rate adjusted to US 2000 standard population, was 10,412 cervical; 4,637 oropharyngeal; 2,371 anus; 1,153 vulva; 385 vagina; and 298 penis.12 It has also been reported that the annual number of noncervical cancers attributable to HPV approximates that of cervical cancer, and the annual number of noncervical cancers attributable to HPV in men approximates that among women.30 The Surveillance, Epidemiology, and End Results program (SEER) of the National Cancer Institute studied incidence and survival of HNSCC in the United States from 1973 to 2004 and determined that there was an increasing incidence of HPV-related cancers in the base of the tongue, palatine tonsil, and oropharynx but a decreasing incidence of HPV-unrelated cancers in the tongue, gingiva, floor of mouth, palate, and "other" mouth.12 The mean age at diagnosis by calendar time for HPV-related carcinoma, 61.1 years, compared to HPV-unrelated carcinoma, 64.5 years, was statistically significant and indicated a 0.5 years decrease per decade for the former and a 0.7 years increase per decade for the latter. From 1973 to 2005, the proportion of all HNSCC of the oropharynx increased from 18% to 32%. The Swedish Cancer Registry reported tonsillar cancer HPV prevalence by calendar period increased from 23% in 1970-1979 to 93% in 2006-2007.31 Lastly, HPV in HNSCC incidence projections (2010 to 2030) indicate a continued decline in the oral cavity and larynx but a steep increase in the oropharynx.12 Thus, behavioral epidemiologists hypothesize that the changing sexual behaviors beginning in the 1960s led to increased HPV exposure. Currently, it is unknown what impact the HPV vaccines Gardasil and Cervarix will have on the future incidence rates of the subset of HPV-associated OPSCC (especially type 16), although many researchers hypothesize a decrease will be observed in approximately 20 years. Also, some researchers are investigating these or similar future vaccines for potential use in treatment of HPV-associated OPSCC.
Studies have revealed that HPV-positive OPSCC are more responsive to radiation treatment than HPV-negative tumors but not necessarily with chemoradiation or platinum induction chemotherapy.32 Tumors caused by HPV infection are known to have less genetic damage than HPV-negative tumors, which enhances response to radiation therapy.33 HPV positivity accounts for approximately 10% of relative survival benefit, and tumor HPV status is the single greatest independent prognostic factor for head and neck cancer. The relative survival for HPV-positive patients is independent of therapy, but the absolute survival difference between HPV-positive and HPV-negative at 5 years is consistently 30% or greater.32 The mechanism underlying this favorable prognosis may involve the combined effect of immune surveillance to viral-specific tumor antigens, an intact apoptotic response to radiation, and the absence of field cancerization. Improved survival is multifactorial due to other favorable factors and improved local regional control. For example, those patients whose tumors are HPV-positive but who also use alcohol and smoke tobacco have a diminished survival compared to those who do not smoke or drink.34 Therefore, tumor HPV status should be determined for all patients diagnosed with OPSCC;
this is presently best accomplished by a combination of p16 immunohistochemistry stain and HPV in situ hybridization.
In 2009 the American Dental Association's Council on Scientific Affairs commented that before specific guidance regarding HPVs and OPSCC and OCSCC can be provided to patients, considerable more research is needed. For example, questions must be answered related to the natural history of oral HPV, transmission risks, screening/testing, and the predictive value of a positive HPV test for the subsequent development of oropharyngeal cancer.35
Summary
In summary, HPV-positive OPSCC is a distinct disease entity and oral HPV-16 infection is the principal risk factor, conferring an approximate 15-fold increase in risk. Although dentists are trained to perform a thorough intraoral examination of the oral cavity proper both by direct vision and palpation, it is now increasingly important that they also ensure similar careful and thorough examination of the oropharynx. Despite the fact that early HPV-associated oropharyngeal carcinoma may not be clinically detectable, other signs and symptoms can be present, including localized pain/swelling, sore throat, dysphasia, referred pain to the ipsilateral ear, and cervical lymphadenopathy. Therefore, for earlier detection of OPSCC, dentists should also perform a thorough visual and tactile soft-tissue extraoral examination of the head and neck on all adults, not just those with historical traditional risk factors.
Acknowledgement
The author thanks Maura L. Gillison, MD, PhD, for providing reference material based on her symposium presentation at the annual American Academy of Oral and Maxillofacial Pathology meeting, San Juan, Puerto Rico, April 29, 2011.
References
1. Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78-81.
2. Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
3. National Cancer Institute. Lip and Oral Cavity Cancer Treatment (PDQ®). Bethesda, MD: National Institutes of Health; date last modified July 20, 2010. http://cancer.gov/cancertopics/pdq/treatment/lip-and-oral-cavity/HealthProfessional. Accessed June 5, 2011.
4. National Cancer Institute. Oropharyngeal Cancer Treatment (PDQ®). Bethesda, MD: National Institutes of Health; date last modified July 20, 2010. http://www.cancer.gov/cancertopics/pdq/treatment/oropharyngeal/Patient/page1. Accessed June 5, 2011.
5. Alani RM, Munger K. Human papillomaviruses and associated malignancies. J Clin Oncol. 1998;16(1):330-337.
6. Closmann JJ. The human papilloma virus, the vaccines, and oral and oropharyngeal squamous cell carcinoma: what every dentist should know. Gen Dent. 2007;55(3):252-254.
7. National Cancer Institute. Fact Sheet 3.20: Human Papillomaviruses and Cancer. Bethesda, MD: National Institutes of Health; date last modified December 12, 2010. http://www.cancer.gov/cancertopics/factsheet/Risk/HPV. Accessed June 5, 2011.
8. Craig G, Johnson N, eds. British Dental Association Occasional Paper: Opportunistic Oral Cancer Screening. 2000;4(6)1-35.
9. Warnakulasuriya S. Causes of oral cancer—an appraisal of controversies. Br Dent J. 2009;207(10):471-475.
10. Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488-1494.
11. Attner P, Du J, Näsman A, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126(12):2879-2884.
12. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612-619.
13. D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Eng J Med. 2007;356(19):1944-1956.
14. Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118(6-7):510-519.
15. Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16(11):1671-1677.
16. Syrjänen S, Lodi G, von Bultzingslowen I, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(suppl 1):58-72.
17. Wilczynski SP, Lin BT, Xie Y, Paz IB. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol. 1998;152(1):145-156.
18. Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58(1):5-13.
19. Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747-753.
20. Balz V, Scheckenbach K, Götte K, et al. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63(6):1188-1191.
21. Wiest T, Schwarz E, Enders C, et al. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21(10):1510-1517.
22. Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(3 pt 1):701-709.
23. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467-475.
24. Mork J, Lie K, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125-1131.
25. Hocking JS, Stein A, Conway EL, et al. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104(5):886-891.
26. Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686-698.
27. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407-420.
28. D'Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263-1269.
29. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709-720.
30. Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 suppl):3036-3046.
31. Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620-2623.
32. Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24(17):2606-2611.
33. Chaturvedi A, Engels E, Pfeiffer R, et al. Human papillomavirus (HPV) and rising oropharyngeal cancer incidence and survival in the United States. J Clin Oncol. 2011;29(suppl; abstract 5529).
34. Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
35. American Dental Association. Statement on Human Papillomavirus and Cancers of the Oral Cavity and Oropharynx. Chicago, IL: ADA Council on Scientific Affairs; April 2009. www.ada.org/1749.aspx. Accessed March 3, 2011.
About the Author
Michael A. Kahn, DDS
Professor and Chairman,
Department of Oral and Maxillofacial Pathology
Tufts University School of Dental Medicine
Boston, Massachusetts