You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Recent developments in dental adhesion and adhesive luting materials has modified and improved clinical dentistry. Previously many complete crown restorations were done with a high degree of enamel present. The advent of the high-speed handpiece and restorations that required greater reduction for both esthetics and strength necessitated the introduction of cements that would seal the dentinal surface and retain the restorations.
The establishment of hybridized dentin serves as synthetic enamel. The hybrid layer substitutes resin for the mineral in the subsurface of mineralized tissue.1,2 Hybridized dentin is a blend of collagen and resin polymers. The dentin has to initially be conditioned or acid-etched, and then the monomers that were placed on the surface polymerize in the matrix of the demineralized dentin. The dentin must be demineralized because mineralized dentin allows minimal diffusion. In addition, the smear layer covering the dentin is only inadequately attached to the dentin and should be removed or modified to allow sufficient monomer diffusion into the demineralized collagen matrix. Due to the hydrophilic character of the matrix, the use of monomers that are both hydrophilic and hydrophobic are required for enhanced adhesion. The hydrophilic nature of the monomer allows its penetration into the collagen matrix to form the collagen-resin hybridized layer. The hydrophobic property enables the interaction with the subsequent hydrophobic resin matrix of the restoration.3 The removal of hydroxyapatite crystals by the etching process creates spaces between the collagen fibrils. If these spaces are not filled, the collagen will collapse and decrease interfibrillar spacing and reduction or elimination of permeability to resin monomers.
History of Dentin Bonding
In 1951, Swiss chemist Dr. Oscar Haggar developed an acidic glycerophophoric acid dimethacrylate that allowed resin to adhere to dentin.4 The commercial product that utilized this material was Servitron (Amalgamated Dental Co, Ltd, London, United Kingdom). Buonocore5 created acid-etched enamel using phosphoric acid to increase resin–enamel bond strength. Gwinnett6 used an electron microscope to view the interface created and found the adhesive resins had penetrated the acid-etched enamel prisms and surrounded the apatite crystals, forming an acid-resistant layer. This layer was neither enamel nor resin but a hybridization of both materials. Buonocore’s success on enamel unfortunately did not work on dentin due to the inefficient wetting agents present at the time and lack of knowledge of the dentin substrate. Subsequently, a variety of manufacturers used phosphate esters of methacrylic acid, but most of them were intended for placement directly on the weak smear layer.
These monomers minimally penetrated the smear layer and produced bond strengths of only 5 MPa. Tao et al7 demonstrated that the bonds failed on both the resin and dentin sides and the 5 MPa bond strength was actually the cohesive force holding the smear particles together. It was thought that higher bond strengths could only be achieved if the smear layer was removed or modified.8,9 Watanabe et al10 demonstrated a correlation between tensile bond strengths of resin to smear layer. In Japan, Masuhara utilized tri-n-butyl borane (TBB) to launch the polymerization of methyl methacrylate (MMA) to wet ground ivory. The addition of an adhesion promoter, 4-methacryloyloxyethyl trimellitate (4-META), was included. The monomer increased its infiltration but the hybrid layer became too thick. This resulted in defects of unfiltrated collagen fibrils in the subsurface of the subsurface. This demonstrated ivory was a poor substitute as a model for dentin.11 The MMA-TBB resin was also used on acid-etched enamel with good success and was used for orthodontics.12 The curing time proved too long, however, for bonding the plastic brackets to enamel, and when the polymerization rate was increased the bond strength decreased.
Difficulty in Bonding to Dentin
Other than dentinal tubules, mineralized dentin has very few pores that will allow the infiltration of monomers in the time needed for clinical use. Acid-etching of enamel and dentin causes the removal of the mineral phase and increases porosity. The increased porosity allows appropriate monomers to infiltrate the dentinal surface. The establishment of a hybridized dentin is contingent on the permeability of both the dentin substrate and diffusion rate of the applied adhesive monomer. However, if the mineral phase is removed from the collagen in order to allow monomer infiltration, ionic bonding to calcium and phosphate are lost. In addition, after the hydroxyapatite crystal is removed the dentinal matrix can collapse during the conditioning phase resulting in a diminution of interfibrillar arrangement and lack of permeability to resin monomers. When an acid conditioner such as 10% citric acid/3% ferric chloride (10-3) is used, collagen fibrils don’t collapse; this allows dry bonding so as to minimize the deviation that occurs with wet bonding.13 The addition of 3% ferric chloride in acid conditioning also prevents protein denaturation during the demineralization.14 The dentin primer functions to keep the demineralized dentin wet in order to avoid their collapse. The spaces between the collagen fibrils are maintained so that bonding agents subsequently applied can diffuse into the demineralized dentin to form hybridized dentin.15 The original 4 META/MMA-TBB required PMMA powder in order for the resin to polymerize. Although this increased the film thickness the addition of HEMA after the 10-3 dentin pretreatment as a priming step heightened monomer penetration and bond strength.
Dentin is frequently tainted with remnants of provisional cements, desensitizers, and a variety of irrigants used for root canal treatment. Adhesive systems can be either phosphate or carboxylic acid resins.16 SuperBond C&B (Sun Medical Co Ltd, www.sunmedical.co.jp, marketed in North America by Parkell, Inc as C&B-Metabond, www.parkell.com) is a carboxylic-based acid resin that utilizes an aqueous solution of 10% citric acid/3% ferric chloride (10-3) to eliminate the smear layer and demineralize the dentin. The adhesive monomer present in SuperBond C&B then penetrates the demineralized dentin and forms a hybrid layer. The ferric chloride plays a key role in this process as the polymerization of methyl methacrylate (MMA) is accelerated by ferric ion on the dentin surface and may aid in reducing cure shrinkage stresses.17 The permeability of dentin that had been conditioned with a combination of 10% citric acid and 3% ferric chloride can be high although dried.2 In addition, the ferric ion can possibly subdue the denaturing effect of dentin collagen that had been treated with a pretreatment acid.18 The ferric chloride hindered the collapse of the demineralized dentin, and 4-META, which contains both hydrophobic and hydrophilic groups, enhances monomer impregnation. Usually collagen is exposed as a consequence of dentin demineralization and 4-META/MMA-TBB resin will provide protection for this network, but any areas not protected may undergo hydrolysis. In addition, over time the tensile bond strength between 4-META/MMA-TBB resin and dentin that had been treated with 10-3 will decrease. When NaOCl in conjunction with phosphoric acid has been used to remove the inorganic and organic constituents of dentin, the problems associated with hydrolytic attack seem to decrease, possibly as a result of the eradication of collagen from the dentin surface.18 Unfortunately, the use of NaOCl seems to impede the polymerization of 4-META/MMA-TBB resin. The use of 10% ascorbic acid (10% sodium ascorbate) reverses this detrimental effect. This is important because a 5% solution of NaOCl is routinely used in endodontic procedures. The ascorbic acid can be used for cleaning, etching, and to increase the rate of polymerization, and is comparable to ethylenediaminetetraacetic acid (EDTA) as a cleansing solution.19
The use of 4-META/MMA-TBB for sealing root apices has also been proposed because of its adhesive properties, little cytotoxicity, high level of polymerization even under moist conditions, and a high degree of biocompatibility upon curing. It actually has been shown that only minor change in bond strength occurs even when the dentin is contaminated with blood.19 This would be helpful in endodontic surgery. The brush-and-dip method of application allows easy application without a cavity preparation. Some clinical studies have illustrated the use of 4-META/MMA-TBB resin as a root-end sealant after apicoectomy. It also has been used to seal vertical fractures, and they don’t reoccur.20
4-META/MMA-TBB resin has also been used as a root canal cement. MetaSeal™ (Parkell, Inc) contains a liquid component hydrophilic monomethacrylates and 4-META and other dimethacrylates. The powder portion contains zirconium oxide and amorphous silica as fillers and polymerization initiators. Sodium hypochlorite (NaOCL) and ethylenediamine tetra-acetic acid (EDTA) is recommended for removing the root canal’s smear layer, thus allowing the diffusion of the hybrid root seal into the radicular dentin. However, Pinna et al21 indicated hybridization can occur only if EDTA is used as a calcium chelator and demineralizing agent after use of NaOCL. If NaOCL is used as the final rinse the demineralized collagen matrix created by the EDTA hybrid layer will not be present. The 4-META in the sealer increases the resin infiltration into the preformed collagen matrix.21
Metal Adhesion
The resin/metal interface is the area where most resin-bonded prostheses fail. The failure is usually caused by the type of adhesive and the alloy used.22 No matter what adhesive is chosen, base-metal alloys usually provide the best result. 23 The bond strength of cast restorations decreased as the nobility increased.23 Base-metal alloys are more reactive than high-noble alloys, and the higher bond strengths may be a result of the higher free-surface energy and the oxide potential of their surface.24 Mechanical retention in addition to chemical adhesion of the resin composite luting material is required as the dental prostheses undergo intraoral load and thermal cycling. Combining both lessens the occurrence of separation at the resin-metal interface. Sen et al25 and others demonstrated comparable bonds between base-metal alloys and Panavia-Ex and Superbond.
Denture-based resins are usually attached to removable partial dentures (RPDs) by loops, struts, beads, or mesh. However, this mechanical attachment allows leaching of oral fluids at the interface between metal and resin. Deterioration and discoloration of the appliance is a frequent result. If the bond between the denture-based resin and alloy were increased, the design of the denture could be modified and bacteria retention and discoloration eliminated. An acrylic-resin denture-based material that contains 4-META (MetaDent, Sun Medical Co Ltd) incorporates 5% 4-META in the monomer and may be recommended where insufficient mechanical retention could be obtained.26 A denture base resin, MetaDent was used in conjunction with the Rocatec™ System (3M ESPE) and silane to create a bond strength of 23.9 MPa.27
A 4-META bonding agent (Amalgambond®, Parkell, Inc) can also be used to repair and increase the retention of an amalgam restoration. Amalgambond® Plus includes a high-performance additive (HPA) to be used with amalgam that is condensed into the material while it is wet. Similar products available for this purpose include: RESINOMER™ Amalgam Bonding/Luting System (BISCO); Scotchbond Multi-Purpose (3M ESPE); Panavia 21 (Kuraray).
To repair an old amalgam the surface is roughened with a carbide bur or a microetching unit (Danville Materials, www.danvillematerials.com). The dentin activator is applied to the exposed dentin for 30 seconds, rinsed with water and then dried. The adhesive agent is applied to the bonding surface and air-dried for 30 seconds. Three drops of base and one drop of catalyst are mixed with one scoop of HPA powder and applied onto the surface to be bonded. The amalgam is then condensed into the wet mixture. The amalgam being condensed into the polymerizing dentin leaves no gap as the cavity walls are sealed with resin-dentin hybridization. These bonded amalgam restorations allow less microleakage and aid in caries prevention.28
Case Report
The patient presented tooth No. 14 with a large amalgam and a fractured lingual cusp (Figure 1). Options presented to the patient included a porcelain-fused-to-metal (PFM) crown, an all-ceramic crown, or a composite onlay, which would conserve as much tooth structure as possible. The composite onlay was selected and the tooth was prepared for the onlay (Figure 2). An impression of the preparation captured the margins of the preparation despite the apical position of the original amalgam (Figure 3). The provisional restoration was removed, and the remaining temporary cement and debris was removed from the tooth with a microetcher and 50-µm aluminum oxide. The preparation was rinsed with water, lightly air-dried and blotted with a cotton pellet to remove excess water. The restoration was tried-in and the margins and contacts verified. The restoration was then bonded with SEcure™ Adhesive Resin Cement (Parkell, Inc). SEcure™ Primer (containing 4-META) was dispensed in a well and one generous coat was applied to the preparation (Figure 4). It was left on for 10 to 20 seconds and air was blown gently across the liquid for 5 seconds.
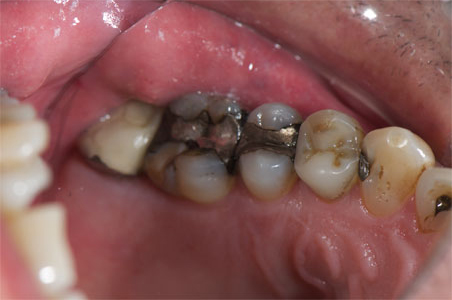 Figure 1 Tooth No. 14 with an extensive amalgam and a fractured lingual cusp. | 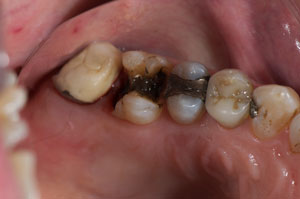 Figure 2 Tooth No. 14 was prepared for an onlay. |
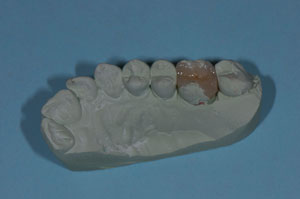 Figure 3 Impregum™ impression delineating margins of preparation. | 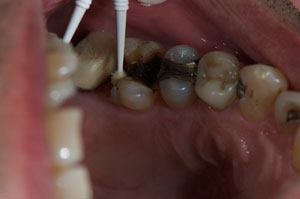 Figure 4 SEcure™ primer was placed on the preparation. |
The cap is removed from the SEcure™ resin cement syringe and bled to verify cement is flush with the orifices of both barrels. A 4:1 mixing tip is placed and then the syringe is bled again and placed on the restoration (Figure 5). The tips are color-coded; the 4:1 tip is brown with an orange mixer.
The restoration was then fully seated and excess that extruded from the margins removed with a rubber tip. While the restoration was being held in place dental floss was run interproximally to remove excess in this area. Light-curing for 2 to 3 seconds allowed more material to be pealed away. After a 3 to 5 minute wait for complete self-cure, the occlusion was adjusted (Figure 6).
 Figure 5 SEcure™ resin cement was placed on the restoration. |  Figure 6 The restoration was luted into position. |
Conclusion
Major accomplishments in adhesion have occurred in the past 50 years since Buonocore’s work on enamel bonding. Dental adhesives and cements are a complex mixture of ingredients designed to provide retention for a variety of materials to tooth structure. These materials must interact with enamel, dentin, and the restorative material. The development of 4-META has led to a wide variety of adhesives and cements over the past 30 years, but all have had the same basic ingredients since the beginning and provided excellent consistency.29
References
1. Nakabayashi N, Koijima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-273.
2. Nakabayashi N, Pashley DH. Evolution of Dentin-Resin Bonding. In: Hybridization of Dental Hard Tissues. Tokyo, Japan: Quintessence Publishing Co; 1998:1-20.
3. Vaidyanathan TK, Vaidtanathan J. Recent advances in the theory and mechanism of adhesive resin bonding to dentin: A critical review. J Biomed Mater Res B Appl Biomater. 2009;88(2):558-578.
4. Kramer IR, McLean JW. Alterations in the staining reactions of dentine resulting from a constituent of a new self-polymerizing resin. Br Dent J. 1952;92:150-153.
5. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34(6):849-853.
6. Gwinnett AJ, Matsui A. A study of enamel adhesives. The physical relationship between enamel and adhesive. Arch Oral Biol. 1967;12(12):1615-1620.
7. Tao L, Pashley DH, Boyd L. Effect of different types of smear layers on dentin and enamel bond strengths. Dent Mater. 1988;4(4):208-216.
8. Eick JD, Cobb CM, Chappell RP, et al. The dentinal surface: its influence on dentinal adhesion. Part I. Quintessence Int. 1991;22(12):967-977.
9. Eick JD, Cobb CM, Chappell RP, et al. The dentinal surface: its influence on dentinal adhesion. Part II. Quintessence Int. 1992;23(1):43-51.
10. Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res. 1994;73(6):1212-1220.
11. Ashizawa M, Watanabe I, Nakabayashi N. Adhesion of MMA-TBB resins to ground ivory. Examination of hybrid layer created in the subsurface. J Jpn Dent Mater. 1992;11:860-865.
12. Miura F, Nakagawa K, Masuhara E. New direct bonding system for plastic brackets. Am J Orthod. 1971;59(4):350-361.
13. Nakabayashi N, Hiranuma K. Effect of etchant variation on wet and dry dentin bonding primed with 4 META/acetone. Dent Mater. 2000;16(4):274-279.
14. Nakabayashi N, Watanabe A, Gendusa NJ. Dentin adhesion of “modified” 4-META/MMA-TBB resin: function of HEMA. Dent Mater. 1992;8(4):259-264.
15. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-273.
16. Soeno K, Suzuki S, Taira Y, Atsuta M. Improvement of the bond strength of 4-META/MMA-TBB resin to collagen-depleted dentin. J Biomed Mater Res B Appl Biomater. 2005;73(1):104-108.
17. Imai Y, Kadoma Y, Kojima K, et al. Importance of polymerization initiator systems and interfacial initiation of polymerization in adhesive bonding of resin to dentin. J Dent Res. 1991;70(7):1088-1091.
18. Soeno K, Taira Y, Jimbo R, Sawase T. Surface treatment with ascorbic acid and ferric chloride improves the micro-tensile bond strength of 4-META/MMA-TBB resin to dentin. J Dent. 2008;36(11):940-944.
19. Sugaya T, Noguchi H, Hasegawa Y, Tanaka Y, Kawanami M. Clinical evaluation of 4-META/MMA-TBB resin as a root-end sealant following apicoectomy. Jpn J Conserv Dent. 2002;45:62–67.
20. Nakabayashi N, Watanabe A, Ikeda W. Intra-oral bonding of 4-META/MMA-TBB resin to vital human dentin. Am J Dent. 1995:8(1):37-42.
21. Pina L, Loushine RJ, Bishop FD Jr, et al. Hybrid root seal (MetaSEAL) creates hybrid layers in radicular dentin only when EDTA is used as the final rinse. Am J Dent. 2009;22(5):299-303.
22. Moulin P, Degrange M, Picard B. Influence of surface treatment on adherence energy of alloys used in bonded prosthetics. J Oral Rehabil. 1999;26(5):413-421.
23. Aquilino SA, Diaz-Arnold AM, Piotrowski TJ. Tensile fatigue limits of prosthdodontic adhesives. J Dent Res. 1991;70(3):208-210.
24. Verzijden CW, Feilzer AJ, Creugers NH, Davidson CL. The influence of polymerization shrinkage of resin cements on bonding to metal. J Dent Res. 1992;71(2):410-413.
25. Sen D. Nayir E, Pamuk S. Comparison of the tensile bond strength of high-noble, noble, and base metal alloys bonded to enamel. J Prosthet Dent. 2000;84(5):561-566.
26. Jacobson TE, Chang JC, Keri PP, Watanabe LG. Bond strength of 4-META acrylic resin denture base to cobalt chromium alloy. J Prosthet Dent. 1988;60(5):570-576.
27. NaBadalung DP, Powers JM, Connelly ME. Comparison of bond strengths of denture base resins to nickel-chromiumn beryllium removable partial denture alloy. J Prosthet Dent. 1997;78(6):566-573.
28. Charlton DG, Moore BK, Swartz ML. In vitro evaluation of the use of resin liners to reduce microleakage and improve retention of amalgam restorations. Oper Dent. 1992;17(3):112-119.
29. Chang JC, Hurst TL, Hart DA, Estey AW. 4-Meta use in dentistry: a literature review. J Prosthet Dent. 2002;87(2):216-224.

