You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by a chronic and remitting disease course with varying clinical manifestations.1 The heterogeneous nature of SLE results in a wide range of disease presentation, and the disorder has the potential to manifest in nearly every patient organ system.2,3 To date there is no known cure for SLE, and therapeutic goals revolve around managing adjunctive clinical symptoms, secondary organ damage, and disease flare-ups.4 The heterogeneous course of SLE establishes its own unique set of dental concerns and considerations for the dental practitioner managing a patient diagnosed with SLE. The approach to dental treatment will depend on the severity of disease presentation and the therapeutic regimen being used to curtail the varying effects experienced by each patient. This article highlights key information to help dental practitioners treat and manage patients with SLE.
Medical Background
Symptoms and Diagnosis
Clinical symptoms of SLE are numerous and can vary drastically among patients.5 The disease has a disproportionate predilection for females of childbearing age compared to the male counterpart while also having an increased prevalence and incidence among certain groups of people.2,3,6,7 Ethnic populations with a more marked affliction of SLE include those of African, Asian, and Hispanic heritage.6,7 Lupus erythematosus can be categorized as a collection of autoimmune connective tissue disorders where cellular constituents, including those such as the nucleus and the cytoplasmic membrane, are targeted by circulating autoantibodies.3 This disease process may afflict all parts of the body. Although the etiology of SLE remains unknown, researchers are continually increasing their understanding of this multifactorial disease process. It has been suggested that deposits of antigen antibody complexes play a part in tissue damage, which is characteristic to lupus erythematosus.3
Diagnosing SLE can be challenging due to its broad spectrum of manifestations, as symptoms can mimic other disease processes.8 On average, it takes approximately 4 years from a patient's initial onset of symptoms to diagnose SLE, and patients will see an average of three different healthcare professionals before being properly diagnosed with the disease.8 A diagnosis of SLE is formed from a combination of clinical signs and symptoms, along with various positive serologies and biopsies.9
Multiple SLE classification systems exist, however most are used only for research purposes and epidemiological data as they typically are not sensitive enough to confirm a diagnosis.9 In 1999 the American College of Rheumatology (ACR) developed 11 criteria for SLE classification, requiring patients to have a minimum of four out of 11 reported manifestations.10 In 2012 the Systemic Lupus International Collaborating Clinics (SLICC) revised the ACR's SLE classification criteria to include 17 criteria for diagnosis (Table 1).11 The SLICC classification system requires patients to have at least four out of the 17 criteria for SLE classification, and patients typically need a minimum of one clinical and immunologic criterion.11 Clinicians can use these classification systems to aid in diagnostic clinical judgment; however, they should keep in mind that a diagnosis is highly individualized, and classifications are not.10
Currently there is no single serologic test that can diagnose SLE, nor is there a current test that can reliably measure SLE disease activity.12 However, approximately 97% of SLE patients present with abnormally high antinuclear antibody levels.13 The most accurate urine test to assess for SLE is the 24-hour urine protein test, which identifies any proteins in the urine, indicating abnormal kidney function.14 Kidney biopsy remains the gold standard for diagnosing SLE kidney manifestations, and skin biopsies of SLE dermal lesions can be critical for diagnosis if clinical tests remain inconclusive.15,16Although there are no current diagnostic imaging tests that are universally accepted for diagnosis, imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT) scans can assist clinicians in determining the extent and severity of disease in patients currently diagnosed with SLE.17
The multitude of varied clinical manifestations of SLE can affect nearly all organs and tissues.18 The disease course for SLE differs among patients and can include periods of flare-ups and remissions.19 Certain irritating agents known as triggers can cause an increase in a patient's SLE symptoms and should be avoided.20 Common triggers include stress, natural and artificial ultraviolet rays, infections, and sun-sensitizing drugs such as tetracyclines and sulpha drugs.20 Patients with SLE can experience generalized symptoms that may include but are not limited to fatigue, fever, malaise, headache, weight loss, and anorexia.18 Many SLE patients develop skin manifestations. Individuals may present with coin- or disc-shaped lesions,19 which typically appear on the face, scalp, and sun-exposed areas.20 These lesions consist of plaques that are erythematous in nature and are typically covered in epidermal scales that can extend into hair follicles.20 Patients can also develop the hallmark bilateral malar rash appearing on the nose and cheeks in the shape of a butterfly.19
Joint involvement is common in patients with SLE and is reported in up to 95% of patients with SLE at some point in the course of their disease.18 Principal musculoskeletal manifestations related to the disease process are arthralgia and arthritis; however, uncommon manifestations such as osteonecrosis, fibromyalgia, and fragility fractures have been reported.19 Renal manifestations in SLE are termed lupus nephritis, and renal involvement is the most important factor influencing a patient's overall prognosis.21 Hematological manifestations are common in SLE patients and may be further worsened by immunosuppressive agents.22 Lymphopenia, neutropenia, and thrombocytopenia are all potential complications of SLE.22 Neutropenia, defined as <1.9 x 10^9 neutrophils/L, is typically asymptomatic and is responsible for recurrent infections and immunosuppression in SLE patients.22 Lymphopenia is defined as <1.5 x 10^9 lymphocytes/L and is associated with an increased risk of infection.22 Thrombocytopenia, defined as <100 x 10^9 platelets/L, can cause prolonged bleeding in patients, gingival bleeding, hematuria, and excessive contusions.22
Treatment Approaches
Currently there is no known cure for SLE. As noted earlier, treatment goals are typically centered around managing SLE symptoms, preventing further organ damage, and minimizing disease flare-ups.4 Due to the photosensitive nature of SLE, patients are usually advised to reduce sun exposure as much as possible, as this can exacerbate symptoms.4 Lifestyle changes such as a well-balanced diet and regular physical activity can be beneficial in managing patient symptoms.20
Hydroxychloroquine (HCQ), an antimalarial drug, is the most common medication recommended for SLE patients.4 HCQ has been shown to dramatically improve patient symptoms and has few reported side effects, which typically decrease over time.4 One serious adverse reaction of HCQ, however, is retinal toxicity. Additionally, smoking cessation is critical for patients on this medication as cigarette smoke interferes with the effectiveness of antimalarial medication.4
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often given to SLE patients for short-term treatment to decrease systemic inflammation and manage joint pain, headaches, and fever.23 Patients taking long-term NSAIDs need to be monitored for gastric bleeding and ulcers.23 Glucocorticoids (GCs), a class of corticosteroids, are often prescribed for SLE patients but only for short-term use due to their detrimental effects on the body.4 After taking an oral GC such as prednisone patients will notice a marked and rapid improvement in their SLE symptoms.4
Immunosuppressants are an important class of medications in the management of SLE symptoms.24 These drugs are crucial for decreasing flare-ups in SLE patients and can also be used to decrease dosages of other medications with adverse reactions, such as GCs.4 Methotrexate, cyclophosphamide, and azathioprine are commonly prescribed immunosuppressants for SLE patients; these are typically considered if symptoms are being poorly controlled by other pharmacological agents.4
Biologic agents are the drug class with the most recent development in the treatment of SLE.25 Belimumab is a B-cell targeting human monoclonal antibody used in the treatment of SLE.25 Belimumab decreases patient symptoms by inhibiting a cytokine known as B-lymphocyte stimulator, thus decreasing the patient's immune response.25 Belimumab is typically considered for patients with inadequate relief of persistent extra-renal symptoms from first line SLE treatments.4
Dental Background
Oral Findings
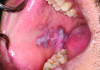
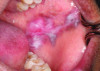
Oral lesions are a common sign of SLE and can manifest in various appearances.26 Approximately 40% of SLE patients experience these lesions, which can be challenging for a practitioner to diagnose.26 The most frequently seen oral lesion in SLE is described as an oral discoid lesion, which typically presents as an ulcer with a scalloped white border displaying keratotic striae and an erythematous center.27 Clinical photographs of a patient with SLE and its associated oral manifestations are shown in Figure 1 and Figure 2.
Other common oral manifestations include erythematous ulcers, verrucous lupus lesions, and hyperkeratotic plaques.27 Aphthous ulcers also may manifest in patients with SLE, however these are usually more common in patients with juvenile-onset SLE when the disease is in an active phase.27 Other rarely reported oral lesions associated with SLE include telangiectasia, and bullae of the buccal mucosa.27 SLE patients with thrombocytopenia can also develop oral petechiae and/or purpura and may experience spontaneous gingival bleeding.27 The vermillion area of the lower lip also has been occasionally reported to be affected; this is known as lupus cheilitis.28 Desquamative gingivitis is another oral lesion associated with SLE. It is an inflammatory lesion of the gingiva that is non-biofilm induced.29 Table 2 provides a list of oral SLE lesions.26-29
Xerostomia is often present in patients with SLE due to the notable decrease in salivary gland function. SLE patients are also prone to developing secondary Sjogren's syndrome, which is dryness of the eyes and mouth in the presence of an autoimmune disorder.30 Xerostomia is reported in more than 75% of SLE patients and can lead to unfavorable oral manifestations. For instance, increased rates of dental caries, periodontal disease, and candidiasis infections (eg, angular cheilitis, exfoliative cheilitis, and oral thrush) have been reported in these individuals. Xerostomia can also lead to dysgeusia, stomatodynia, and glossodynia in SLE patients.31 SLE patients who do not report xerostomia are still at a higher risk of developing the above symptoms due to their compromised immune status.30 Temporomandibular joint disease (TMD) has also been documented in SLE patients.32 TMD manifestations include but are not limited to osteophytes, erosion of condylar bone, and granulation of TMD joint spaces.32
A very rare clinical manifestation of SLE is avascular necrosis (AVN) of the bone, more commonly known as osteonecrosis.33,34AVN can present as joint pain in patients who have previously suffered physical trauma to the area, or who take corticosteroids chronically.33 The most common site for AVN is the femoral head, and symptoms are similar to arthritis.33 Cone-beam computed tomography (CBCT) imaging techniques have been shown to detect AVN in the early stages.35 Another rare condition that has been reported in SLE patients is trigeminal neuralgia, a facial pain disorder that commonly presents with unilateral instantaneous paroxysmal shocks of pain that can affect the upper, middle, or lower thirds of the face.36
Dental Care
Because of the complex therapeutic regimen and nature of SLE, a consultation with the patient's physician is suggested before any dental treatment is rendered.30 When doing so the dental practitioner should establish the severity of disease and overall stability of the patient.37,38 A hematologic profile should be established due to SLE patients potentially having thrombocytopenia and/or leukopenia.38 Approximately 40% of patients with SLE are also found to have antiphospholipid syndrome, an autoimmune disease characterized by an increased likelihood of venous and arterial thrombosis that can occur in any organ.39 Furthermore, a comprehensive list of medications and suggested dental treatment modifications involved in the therapeutic management of these patients should be provided to the dental practitioner so that such treatment modifications and considerations can be taken.38
When a patient presents to a dental setting with a diagnosis of SLE, patient management will vary depending on how and in which organ system(s) the disease presents while also giving consideration to any medications or therapies being used in the treatment of the disease. Immunosuppressive medications, leukopenia, and prolonged steroid therapy can increase the patient's susceptibility to dental-related infections.40 Most common infections include oral candidiasis, but other opportunistic infections have been reported depending on the dose of medication administered, including but not limited to fungal infections, mycobacterium infections, and cytomegalovirus infections.40 Infections within the scope of the dental practitioner should be treated promptly as oral infections can exacerbate and become a systemic problem for the severely immunosuppressed patient.41 Patients on prolonged steroid and immunosuppressive therapies also may have delayed healing.30
Prophylactic antibiotics should be discussed with the patient's healthcare team ahead of invasive dental treatment to avoid negative patient outcomes.30,41,42 Another consideration for clinicians is that steroid therapy, including prednisone, may also cause adrenal suppression for the patient.2,30,37,38 In severe cases of adrenal suppression the patient may experience a life-threatening situation known as adrenal crisis, which requires immediate medical care.38 While steroid supplementation typically is not needed for routine dental treatment, it may be required for patients with severe anxiety or those undergoing invasive surgical procedures and should be discussed with the patient's medical doctor.38
A common class of pharmacologic agents used to treat SLE patients are NSAIDs. NSAIDs elicit anti-inflammatory and pain modulating effects by inhibiting the enzyme cyclooxygenase.30 This may affect the patient's hematologic profile, increasing the patient's susceptibility to bleeding. SLE patients may also present with thrombocytopenia with or without NSAID use, and referral to an oral surgeon may be warranted if the patient's hematologic profile is dually affected. Such a referral might be recommended for highly invasive dental treatments where controlling bleeding is important, such as tooth extraction or implant placement.
Additional considerations during the examination and management of pain or infection in the patient with SLE may be warranted. For example, patients with SLE have an inherently larger propensity to present with malignancies, such as skin squamous cell carcinomas, lymphomas, Kaposi's sarcoma, and other carcinomas.30,38,43 For malignancies with oral presentations the dental practitioner can be a key contributor to early diagnosis and treatment. Regular oral and head and neck examinations are integral to treatment. Patients with SLE may also present with secondary kidney disease.30,41,44-46 Consideration should be given when prescribing pharmacologic agents with kidney-dependent elimination.30,41,44-46 Such drugs include NSAIDs, penicillin, tetracycline, cephalosporins, acetylsalicylic acid, nystatin, and valacyclovir. Alternatives may include clindamycin and acetaminophen.30,41,44-46
Oral lesions commonly resolve if SLE is adequately controlled, and their recurrence may be indicative of an SLE flare-up.30 However, for SLE patients who develop painful oral lesions, low-level diode laser therapy may be considered to reduce recurrence and pain associated with these lesions.47 Mouthrinses may also be prescribed for patients with painful oral ulcers to reduce patient symptoms and increase overall quality of life.48 A common formula ("magic" mouthrinse) consists of one part diphenhydramine 12.5 mg per 5 ml elixir, one part maalox (no substitution), and one part 2% viscous lidocaine.48 Patients can rinse for approximately 1 minute with two teaspoons every 4 to 6 hours as needed to relieve oral symptoms.48 Management of oral lesions can also include the use of 0.05% fluocinonide gel.
Due to SLE patients presenting with an increased caries and periodontal disease risk, the importance of excellent oral hygiene must be stressed to the patient.49 High fluoride toothpaste should be considered.49 Patients suffering from severe xerostomia may also be interested in a pharmacological agent to reduce their symptoms, such as pilocarpine and cevimeline; however, it is important to discuss the addition of this medication with the patient's healthcare team.31
Conclusion
Systemic lupus erythematosus is a multisystem autoimmune disorder with a diverse set of clinical presentations and no current cure. The mainstay of treatment aims to stabilize symptoms and reduce disease flare-ups while also managing treatment-related adverse effects. The heterogeneous nature of disease presentation, complex tissue damage, and multifactorial therapeutic regimen provide challenges to the dental practitioner. Importance lies in dental practitioners familiarizing themselves with the disease's effects on the patient's physiology and establishing treatment modifications, while also having discussions and making necessary referrals within the allied healthcare team. Through thorough dental provider preparation, medically compromised individuals such as those with SLE can be safely treated and managed within the private practice dental setting.
About the Authors
Brookelyn Pettigrew, DDS
Private Practice, Guelph, Ontario, Canada
Nermin Piragic, DDS
Private Practice, Kitchener, Ontario, Canada
Aviv Ouanounou, BSc, MSc, DDS
Associate Professor, Department of Clinical Sciences, Pharmacology and Preventive Dentistry, Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada; Fellow, International College of Dentists; Fellow, American College of Dentists; Fellow, International Congress of Oral Implantologists
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Lo MS. Concepts in lupus pathophysiology: lessons learned from disease across the spectrum. Clin Immunol. 2022;238:109021.
2. Powers DB. Systemic lupus erythematosus and discoid lupus erythematosus. Oral Maxillofac Surg Clin North Am. 2008;20(4):651-662.
3. Part 6: Periodontal pathology. In: Linde J, Lang NP, eds. Clinical Periodontology and Implant Dentistry. 6th ed. Chichester: Wiley-Blackwell; 2015:353-354.
4. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736-745.
5. Barber MR, Drenkard C, Falasinnu T, et al. Publisher correction: global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(10):642.
6. Dall'Era M, Cisternas MG, Snipes K, et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: the California Lupus Surveillance Project. Arthritis Rheumatol. 2017;69(10):1996-2005.
7. Izmirly PM, Wan I, Sahl S, et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol. 2017;69(10):2006-2017.
8. Kernder A, Richter JG, Fischer-Betz R, et al. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: cross sectional analysis of the LuLa cohort. Lupus. 2021;30(3):431-438.
9. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1-13.
10. Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatology (Oxford). 2020;59(suppl 5):v4-v11.
11. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum.2012;64(8):2677-2686.
12. Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13(5):290-297.
13. Wichainun R, Kasitanon N, Wangkaew S, et al. Sensitivity and specificity of ANA and anti-dsDNA in the diagnosis of systemic lupus erythematosus: a comparison using control sera obtained from healthy individuals and patients with multiple medical problems. Asian Pac J Allergy Immunol. 2013;31(4):292-298.
14. Reyes-Thomas J, Blanco I, Putterman C. Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol. 2011;40(3):138-150.
15. Fernandez-Flores A. Skin biopsy in the context of systemic disease [in English, Spanish]. Actas Dermosifiliogr (Engl Ed). 2019;110(9):710-727.
16. Duran E, Yildirim T, Kalyoncu U, et al. AB0347 Renal biopsy in patients with systemic lupus erythematosus: Is it only lupus nephritis? Ann Rheumatic Diseases. 2021;80:1198-1199.
17. Goh YP, Naidoo P, Ngian GS. Imaging of systemic lupus erythematosus. Part I: CNS, cardiovascular, and thoracic manifestations. Clin Radiol. 2013;68(2):181-191.
18. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Bucur). 2011;6(4):330-336.
19. Santos LR, Fasano S, Isenberg DA. Recognition and management of systemic lupus erythematosus. Prescriber. 2019;30(3):13-20.
20. Wallace DJ. Lupus: The Essential Clinician's Guide. 2nd ed. Oxford University Press; 2014:23-44.
21. Wadhwani S, Jayne D, Rovin BH. Lupus nephritis. In: Johnson RJ, Feehally J, Floege J, Tonelli M, eds. Comprehensive Clinical Nephrology. 6th ed. Elsevier; 2019:306-319.
22. Sufian AB, Kashem MA, Biswas S. Pattern of hematological manifestations in patients with systemic lupus erythematosus attending in a tertiary care hospital. J Medicine. 2017;18(2):86-91.
23. Lahita RG, Pal T. Nonsteroidal anti-inflammatory drugs in systemic lupus erythematosus. In: Tsokos GC, ed. Systemic Lupus Erythematosus: Basic, Applied and Clinical Aspects. 2nd ed. Academic Press; 2020:585-589.
24. Pego-Reigosa JM, Cobo-Ibáñez T, Calvo-Alén J, et al. Efficacy and safety of nonbiologic immunosuppressants in the treatment of nonrenal systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken). 2013;65(11):1775-1785.
25. Wise LM, Stohl W. Belimumab and rituximab in systemic lupus erythematosus: a tale of two B cell-targeting agents. Front Med (Lausanne). 2020;7:303.
26. Mailiza F. Oral manifestation of systemic lupus erythematous: a review. Adv Health Sci Res. 2022. https://doi.org/10.2991/ahsr.k.220303.012. Accessed June 27, 2024.
27. Rodsaward P, Prueksrisakul T, Deekajorndech T, et al. Oral ulcers in juvenile-onset systemic lupus erythematosus: a review of the literature. Am J Clin Dermatol. 2017;18(6):755-762.
28. Lugović-Mihić L, Pilipović K, Crnarić I, et al. Differential diagnosis of cheilitis - how to classify cheilitis? Acta Clin Croat. 2018;57(2):342-351.
29. Kranti K, Seshan H, Juliet J. Discoid lupus erythematosus involving gingiva. J Indian Soc Periodontol. 2012;16(1):126-128.
30. Benli M, Batool F, Stutz C, et al. Orofacial manifestations and dental management of systemic lupus erythematosus: a review. Oral Dis. 2021;27(2):151-167.
31. Mortazavi H, Baharvand M, Movahhedian A, et al. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res. 2014;4(4):503-510.
32. Crincoli V, Piancino MG, Iannone F, et al. Temporomandibular disorders and oral features in systemic lupus erythematosus patients: an observational study of symptoms and signs. Int J Med Sci. 2020;17(2):153-160.
33. Doğan I, Kalyoncu U, Kiliç L, et al. Avascular necrosis less frequently found in systemic lupus erythematosus patients with the use of alternate day corticosteroid. Turk J Med Sci. 2020;50(1):219-224.
34. Fernandes EG, Guissa VR, Saviolli C, et al. Osteonecrosis of the jaw on imaging exams of patients with juvenile systemic lupus erythematosus. Rev Bras Reumatol. 2010;50(1):3-15.
35. Fu KY, Li YW, Zhang ZK, Ma XC. Osteonecrosis of the mandibular condyle as a precursor to osteoarthrosis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):e34-e38.
36. Gambeta E, Chichorro JG, Zamponi GW. Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain.2020;16:1744806920901890.
37. Lipsky P, Diamond B. Autoimmunity and autoimmune disorders. In: Longo D, Fauci A, Kasper D, et al, eds. Harrison's Principles of Internal Medicine. 18th ed. McGraw-Hill; 2011:2071-2083, chap 312.
38. Little JW, Miller CS, Rhodus NL. Rheumatologic disorders. Little and Falace's Dental Management of the Medically Compromised Patient. 9th ed. St. Louis, MO: Elsevier; 2018:357-359.
39. Bustamante JG, Goyal A, Rout P, Singhal M. Antiphospholipid syndrome. May 6, 2024. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK430980/. Accessed July 11, 2024.
40. Yang SC, Lai YY, Huang MC, et al. Corticosteroid dose and the risk of opportunistic infection in a national systemic lupus erythematosus cohort. Lupus. 2018;27(11):1819-1827.
41. Albilia JB, Lam DK, Clokie CM, Sándor GK. Systemic lupus erythematosus: a review for dentists. J Can Dent Assoc. 2007;73(9):823-828.
42. Chang YS, Chang CC, Chen YH, et al. Risk of infective endocarditis in patients with systemic lupus erythematosus in Taiwan: a nationwide population-based study. Lupus. 2017;26(11):1149-1156.
43. Menzies S, O'Shea F, Galvin S, Wynne B. Oral manifestations of lupus. Ir J Med Sci. 2018;187(1):91-93.
44. Hajji M, Harzallah A, Kaaroud H, et al. Factors associated with relapse of lupus nephritis: a single center study of 249 cases. Saudi J Kidney Dis Transpl. 2017;28(6):1349-1355.
45. Mok CC, Tse SM, Chan KL, Ho LY. Effect of the metabolic syndrome on organ damage and mortality in patients with systemic lupus erythematosus: a longitudinal analysis. Clin Exp Rheumatol. 2018;36(3):389-395.
46. Weening JJ, D'Agati, VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited [erratum in J Am Soc Nephrol.2004;15(3):835-836]. J Am Soc Nephrol.2004;15(2):241-250.
47. Akerzoul N, Chbicheb S. Low laser therapy as an effective treatment of recurrent aphthous ulcers: a clinical case reporting two locations. Pan Afr Med J. 2018;30:205.
48. Kravitz ND, Crutchfield WE, Miller S, Gill J. Magic mouthwash demystified. J Clin Orthod. 2020;54(8):462-465.
49. Wu AJ. Management of salivary hypofunction in Sjögren's syndrome. Curr Treat Options Rheumatol. 2015;1:255-268.