You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Interceptive orthodontics with maxillary expansion in children, with a focus on pediatric development, is becoming a widespread movement within the dental sleep medicine community. Pediatric orthodontists and pediatric dentists who utilize maxillary and mandibular expansion to improve nasal breathing and tongue placement and to create sufficient space for teeth (including the wisdom teeth) are noticing, in addition to the intended treatment results, unexpected and dramatic improvements in their patients' systemic health. This article will discuss how properly executed dental arch expansion, frenectomy, myofunctional therapy, and nasal hygiene can be the key to mitigating sleep-disordered breathing/obstructive sleep apnea, as well as some of its common comorbidities.
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by intermittent narrowing and obstruction of the airway during sleep, which results in respiratory-related arousals from sleep and decreased oxygen saturation. In children, OSA can have significant effects on their behavior, neurodevelopment, metabolism, and general health. Despite its serious health-related consequences, OSA is a frequently overlooked condition in children, typically having a more subtle presentation than in adults.1 While adult populations manifest much higher rates of excessive daytime sleepiness, children do not.35Children suffering from OSA present with major behavioral issues, inability to focus, and hyperactivity, for example.36
The gold standard for pediatric OSA treatment is tonsil and adenoid removal,2 followed by continuous positive airway pressure (CPAP) if the OSA is not resolved by surgery. CPAP devices, while life-saving, are cumbersome to wear and restrict facial development during the most important years of a child's growth.3 Tonsillectomy is the most common surgery performed in children, with sleep-disordered breathing being the most frequent indication for this procedure,4 while the cure rate for OSA after tonsillectomy for all children ranges from 51% to 83%.4,5 Many children with OSA are not helped by clearing the "breathing straw" through removal of the tonsils and adenoids, often because underdeveloped dental arches and a weak tongue that continues to fall back and obstruct the airway are the cause of their apnea. Furthermore, adenotonsillectomy can be associated with numerous risks and sequelae, including severe postoperative pain, bleeding, dehydration, complications of general anesthesia, and potential long-term adverse effects.4,6
It is therefore crucial that other treatment options to CPAP and surgery be considered and discussed with parents or caregivers of pediatric patients with OSA, including frenectomy, treatments to enhance early jaw development such as myofunctional therapy, maxillary expansion, and even simple interventions such as nasal hygiene. Unfortunately, to date these options have not commonly been discussed with parents of these patients.
Maxillary Expansion for Obstructive Sleep Apnea and Bruxism
Arch expansion is a treatment modality typically used in orthodontics to create space in cases of tooth crowding. Arch expansion can be utilized to improve mastication by correction of the reverse-sequencing chewing cycles.37 Expansion of dental arches has also been shown to correct dysfunction in speech.38
Rapid palatal expansion has been shown to improve pediatric OSA by reducing nasal airway resistance, increasing nasal volume, raising tongue posture, and enlarging the airway,7 and a 12-year follow-up study has confirmed long-term airway stability. In a recent study by Yoon et al, maxillary expansion has also been found to result in a statistically significant reduction in the size of tonsil and adenoid tissue.7,8 These findings highlight the potential for maxillary expansion to enable many pediatric patients with OSA to keep these important lymphatic tissues and avoid their surgical removal.
Until recently, many dental students were taught that maxillary expansion outside of puberty was difficult to achieve and that mandibular expansion was highly unlikely. Historically, mandibular expansion has not been recommended because the mandible has no mid-palatal suture, in contrast to the maxilla.9,10 While some researchers have maintained that the mandibular expansion that is observed is caused only by an increase in dental inclination or is localized to the alveolar process,10-12 other studies have suggested that the width of the mandibular arch can be permanently increased.13,14
One of the most common parasomnias in children is sleep bruxism.15 Numerous studies have been published supporting the correlation between sleep bruxism and OSA.16 Although most of these studies were conducted in adult patients,16 sleep bruxism has been reported to be more common in children than in adults.17 Pediatric sleep bruxism has been shown to have a prevalence rate of 13%-49% with little understanding of viable treatment.17 The watch and wait methods taught in dental schools have little merit given the lack of evidence of spontaneous resolution, and the vast evidence in its correlation to obstructive sleep apnea.34
The dental community needs to reevaluate their approach to bruxism in children. Because bruxism is associated with OSA, a first-line question in the medical history intake form should be whether parents have noticed teeth grinding in their child, so that the child can be properly screened and referred to an airway dentist.18,33 Clinical signs of teeth grinding, such as abfraction lesions and tooth wear, may also be noted during the examination. PSG studies should be performed in all pediatric patients with reported or clinical signs of bruxism.
In the author's practice, children who undergo dental expansion experience complete resolution of their bruxism. While these results are anecdotal, the author recommends this treatment for pediatric patients with this condition, as night guards are not appropriate to use in children whose dentition is still developing.
Mouth-breathing is a common phenomenon in patients with OSA. It is associated with adenotonsillar hypertrophy and is one of the most unhealthy forms of breathing due to the consequences it has on facial skeletal formation.19, 39,40 During mouth-breathing, the air that is inhaled is not warmed, filtered, or humidified, which also inflames the tonsillar tissue.41 Proper breathing (through the nose, with lips sealed) during sleep needs to be ensured for our pediatric patients. If mouth-breathing during sleep is reported, the child should be properly screened via PSG study and evaluated for oral appliance therapy/dental arch expansion by an airway dentist.
Frenectomy, Myofunctional Therapy, and Nasal Hygiene
Tongue posture should be evaluated in patients with mouth-breathing. In an individual with appropriate tongue posture, the tongue is able to sit at the roof of the mouth; correct tongue posture helps ensure proper palate development, promoting palatal expansion naturally. Among patients who are chronic mouth-breathers, the tongue posture tends to be low and arch development is compromised.20
In some children, the tongue is unable to be held in suction to the palate because of a frenum restriction. Unfortunately, there have been some misconceptions surrounding akyloglossia, or tongue-ties. One myth is that the tongue tie will "just grow back" after frenectomy. Also, many parents of children with sleep-disordered breathing or OSA are advised by their doctor that if the child can "stick out their tongue and speak okay," the akyloglossia is not problematic and there is no need to perform a frenectomy. However, it is a serious error to ignore these tethered oral tissues (lip ties, tongue ties, buccal ties), as though the soft tissue plays no role in bone development.21 In the author's opinion, tongue ties tend to be viewed as less important (or at least are treated much differently) than a lower lip tie that causes gum recession, possibly because the damage/recession that the lip tie is causing is readily apparent visually, whereas the tongue tie is not easily seen. Nevertheless, it is important to revise tongue-ties, as this condition can lead to mouth breathing and abnormal development of the oral cavity,22 and is associated with sleep-disordered breathing and underdeveloped jaws.39,40,41,44
Myofunctional therapy has also been emerging in recent years as a complement to lingual frenectomy. Myofunctional therapy plays a critical role from an oral-motor function perspective, aiding in healing post frenectomy, as well as aids in jaw development.43,44 It has been proven that myofunctional therapy by itself can mitigate OSA.23 Furthermore, proper myofunctional therapy, incorporated in the form of rehabilitative tongue exercises after frenectomy, helps ensure that the affected tissues heal beautifully.24
Finally, the importance of nasal hygiene is an easy conversation to have during a dental hygiene visit. For both adults and children, saline nasal sprays improve the amount of oxygen that patients take in with each breath by clearing the nasal passages, which in turn decreases mouth-breathing.25
Comorbidities of OSA: Treatment Considerations
Sleep-disordered breathing is linked to several common medical conditions in children, including migraine and non-specific headache, allergies, and rheumatologic conditions.26 Other noteworthy comorbidities are attention deficit hyperactivity disorder, obesity, and enuresis (bedwetting), some of which can contribute to OSA, and which may be mitigated by its treatment or may indicate that screening for sleep disorders is warranted.
ADHD
Research has shown that children with attention deficit hyperactivity disorder (ADHD) are prone to sleep disorders, and that OSA is linked to ADHD symptoms in these children.27,28Identifying and treating sleep disorders in pediatric patients could represent a life-changing treatment alternative to psychotropic medications for children with ADHD; furthermore, many medical practitioners believe that children with ADHD have been overmedicated.29 Given there is evidence patients apnea hypopnea index improves after dental arch expansion, and the correlation between underdeveloped jaws and ADHD, we must continue to sleep test children suspected of ADHD!45 There long term consequences of medicating children for ADHD are not well understood and published variations in the adherence of the clinical guidelines for managing this condition across the globe.46,47
Obesity
Obesity has long been known to be an important comorbidity of OSA, with a prevalence nearly twice that of normal-weight adults.30 However, although less common in normal-weight adults, OSA can occur even in underweight individuals, as the author has found through home sleep apnea tests administered to adults. With the increasing prevalence of childhood obesity, an emergence of OSA in pediatric patients with obesity has been observed,31and children with obesity should therefore be administered sleep studies. The prevalence of OSA in children with obesity is 44.6%.48Many adult patients believe weight loss alone will "cure" them of their OSA; however, while weight loss has been beneficial in reducing OSA for some,that is not the case for all.49,50 Also, in the author's experience, often patients believe that it is necessary to lose weight before they can begin CPAP or oral appliance therapy (OAT). On the contrary, anyone struggling to lose weight or who notices unexpected weight gain/central fat should undergo sleep screening. Sleep apnea has no "body type," so no opportunity to screen for sleep disorders should be missed, even in patients who are underweight or appear to be "fit."
Enuresis
In a study by Karen Davidson et al in the Journal of Sleep Disorders & Therapy, enuresis was found to be reduced by 97.3% in children who used oral appliance therapy, and the mean number of days for resolution or improvement of bedwetting was 79.7 days.32 The appliances used in this study consisted of myofunctional tooth-positioner appliances which aim to induce nasal breathing and expand the palate by proper directing tongue placement to the roof of the mouth. This study was critical in demonstrating that low oxygen/apnea contribute to nocturnal enuresis, in addition to showing the efficacy of oral appliance therapy in treating this condition. Typical bedwetting treatments have much longer resolution times.51 In the author's opinion, that is because many in the medical community (and many parents) tend to have limited understanding that sleep-disordered breathing/OSA and bedwetting are correlated. If the correlation were better known, sleep studies would be more routinely administered in children with bedwetting.
Maxillary Expansion as an Alternative to Bicuspid Extractions
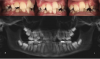
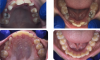
Bicuspid extractions are commonly performed in orthodontic treatment. However, such extractions can lead to a variety of problems, including inadequate space for the tongue. Skeletal & facial discrepancies are noted in the literature when comparing extraction to non-extraction cases with evidence that it does affect the pharyngeal airway size.52,53 In some cases, bicuspid extraction as a treatment approach can be likened to "the shoe is too small so we cut off a toe." Finding alternative approaches to extraction should be a priority for pediatric dentists. In the author's experience, even in some cases of severe crowding, bicuspid extractions were rendered unnecessary because mandibular expansion was utilized instead. In these cases, the parents had been previously offered no other options besides serial extractions of primary teeth followed by removal of all four bicuspids (Figure 1 through Figure 5).
Conclusion
Pediatric sleep medicine is currently plagued by the inefficiencies caused by long wait times for PSG studies, while patients' parents or caregivers frequently are given little to no information on treatment options for OSA other than CPAP therapy and surgery. Dental arch expansion, frenectomy, myofunctional therapy, and even simple interventions such as nasal hygiene are not commonly discussed with patients' parents. Children with sleep-disordered breathing suffer not only from the complications and long-term effects of OSA, but from comorbidities linked to OSA such as ADHD and enuresis, which can be easily mitigated by proper airway management. The use of simple dental appliances for arch expansion and other noninvasive and nonpharmacologic interventions that promote proper oxygenation while preserving important lymphatic tissues can be life-changing for these children. The dental community needs to reevaluate their mindset and approach to pediatric OSA. It is the responsibility of pediatric orthodontists and pediatric dentists to examine the new research surrounding maxillary expansion and interceptive orthodontics to treat OSA and sleep-disordered breathing, so that the current epidemic of sleep disorders can be prevented for generations to come.
References
1. Kansagra S, Vaughn B. Pediatric sleep apnea: Five things you might not know. Neurol Clin Pract. 2013;3(4):321-325.
2. Reckley LK, Fernandez-Salvador C, Camacho M. The effect of tonsillectomy on obstructive sleep apnea: an overview of systematic reviews. Nat Sci Sleep. 2018;10:105-110.
3. Roberts SD, Kapadia H, Greenlee G, Chen ML. Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions. J Clin Sleep Med. 2016;12(4):469-475.
4. Schneuer FJ, Bell KJ, Dalton C, Elshaug A, Nassar N. Adenotonsillectomy and adenoidectomy in children: the impact of timing of surgery and post-operative outcomes. J Paediatr Child Health. 2022;58(9):1608-1615. doi:10.1111/jpc.16052
5. Hairston TK, Links AR, Harris V, et al. Evaluation of parental perspectives and concerns about pediatric tonsillectomy in social media. JAMA Otolaryngol Head Neck Surg. 2019;145(1):45-52.
6. Uwiera TC. Considerations in surgical management of pediatric obstructive sleep apnea: tonsillectomy and beyond. Children (Basel). 2021;8(11):944.
7. Yoon A, Abdelwahab M, Bockow R, et al. Impact of rapid palatal expansion on the size of adenoids and tonsils in children. Sleep Med. 2022;92:96-102.
8. Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med. 2015;16(8):933-935.
9. Zhao B, Zhao G, Shen T, et al. A pilot study of mandibular expansion in combination with a fixed-appliance for increasing the effective space of the mandibular arch: Finite element analysis and three-dimensional cone-beam computed tomography. Medicine (Baltimore). 2021;100(8):e24869.
10. Quinzi V, Mummolo S, Bertolazzi F, Campanella V, Marzo G, Marchetti E. Comparison of mandibular arch expansion by the schwartz appliance using two activation protocols: a preliminary retrospective clinical study. J Funct Morphol Kinesiol. 2020;5(3):61.
11. Tai K, Hotokezaka H, Park JH, et al. Preliminary cone-beam computed tomography study evaluating dental and skeletal changes after treatment with a mandibular Schwarz appliance. Am J Orthod Dentofacial Orthop. 2010;138(3):262.31-262.311.
12. Raucci G, Pachêco-Pereira C, Elyasi M, d'Apuzzo F, Flores-Mir C, Perillo L. Short- and long-term evaluation of mandibular dental arch dimensional changes in patients treated with a lip bumper during mixed dentition followed by fixed appliances. Angle Orthod. 2016;86(5):753-760.
13. Milad SA, Hussein FA, Mohammed AD, Hashem MI. Three-dimensional assessment of transverse dentoskeletal mandibular dimensions after utilizing two designs of fixed mandibular expansion appliance: a prospective clinical investigation. Saudi J Biol Sci. 2020;27(2):727-735.
14. Mahendra TVD, Mulakala V, Anoosha M. Mandibular expansion appliances and their activation protocols. Taiwanese Journal of Orthodontics. 2022;34(3):138-144.
15. Laberge L, Tremblay RE, Vitaro F, Montplaisir J. Development of parasomnias from childhood to early adolescence. Pediatrics. 2000;106(1 Pt 1):67-74.
16. Martynowicz H, Gac P, Brzecka A, et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J Clin Med. 2019;8(10):1653.
17. Bulanda S, Ilczuk-Rypuła D, Nitecka-Buchta A, Nowak Z, Baron S, Postek-Stefańska L. Sleep bruxism in children: etiology, diagnosis, and treatment - a literature review. Int J Environ Res Public Health. 2021;18(18):9544.
18. Li D, Kuang B, Lobbezoo F, de Vries N, Hilgevoord A, Aarab G. Sleep bruxism is highly prevalent in adults with obstructive sleep apnea: a large-scale polysomnographic study. J Clin Sleep Med. 2023;19(3):443-451.
19. McKeown P, Macaluso M. Mouth breathing: physical, mental and emotional consequences. Oralhealth website. https://www.oralhealthgroup.com/features/mouth-breathing-physical-mental-emotional-consequences/ Published March 9, 2017. Accessed May 31, 2023.
20. Chen W, Mou H, Qian Y, Qian L. Evaluation of the position and morphology of tongue and hyoid bone in skeletal Class II malocclusion based on cone beam computed tomography. BMC Oral Health. 2021;21(1):475. Published 2021 Sep 27.
21. Le Révérend BJ, Edelson LR, Loret C. Anatomical, functional, physiological and behavioural aspects of the development of mastication in early childhood. Br J Nutr. 2014;111(3):403-414.
22. Guilleminault C, Huseni S, Lo L. A frequent phenotype for paediatric sleep apnoea: short lingual frenulum. ERJ Open Res. 2016;2(3):00043-2016.
23. Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38(5):669-675.
24. Aikumar S, Srinivasa L, Kennedy Babu, et al. Laser-assisted frenectomy followed by post-operative tongue exercises in ankyloglossia: a report of two cases. Cureus. 2022;14(3):e23274.
25. Murdoch Childrens Research Institute. Simple nasal spray significantly reduces snoring and breathing difficulties in children. Science News website. https://www.sciencedaily.com/releases/2023/01/230117110505.htm. Published January 17, 2023. Accessed May 31, 2023.
26. Lewandowski AS, Ward TM, Palermo TM. Sleep problems in children and adolescents with common medical conditions. Pediatr Clin North Am. 2011;58(3):699-713.
27. Yin H, Yang D, Yang L, Wu G. Relationship between sleep disorders and attention-deficit-hyperactivity disorder in children. Front Pediatr. 2022;10:919572.
28. Wu J, Gu M, Chen S, et al. Factors related to pediatric obstructive sleep apnea-hypopnea syndrome in children with attention deficit hyperactivity disorder in different age groups. Medicine (Baltimore). 2017;96(42):e8281.
29. Tatlow-Golden M, Prihodova L, Gavin B, Cullen W, McNicholas F. What do general practitioners know about ADHD? Attitudes and knowledge among first-contact gatekeepers: systematic narrative review. BMC Fam Pract. 2016;17(1):129.
30. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711-719.
31. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251-265.
32. Parker Davidson K. The association of nocturnal enuresis and breathing disorders in children with sleep disordered breathing: a retrospective review of pediatric cases treated with a preformed monoblock oral appliance. J Sleep Disord Ther. 2022;11:359.
33. Ferreira NM, Dos Santos JF, dos Santos MB, Marchini L. Sleep bruxism associated with obstructive sleep apnea syndrome in children. Cranio. 2015 Oct;33(4):251-5. doi: 10.1080/08869634.2015.1097299. Epub 2015 Dec 29. PMID: 26715296.
34. Khoury S, Rouleau GA, Rompré PH, Mayer P, Montplaisir JY, Lavigne GJ. A significant increase in breathing amplitude precedes sleep bruxism. Chest. 2008 Aug;134(2):332-337. doi: 10.1378/chest.08-0115. Epub 2008 May 19. PMID: 18490400.
35. Gozal D, Wang M, Pope DW Jr. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001 Sep;108(3):693-7. doi: 10.1542/peds.108.3.693. PMID: 11533338.
36. Kang M, Mo F, Witmans M, Santiago V, Tablizo MA. Trends in Diagnosing Obstructive Sleep Apnea in Pediatrics. Children (Basel). 2022 Feb 24;9(3):306. doi: 10.3390/children9030306. PMID: 35327678; PMCID: PMC8947481.
37. Piancino MG, Cordero-Ricardo M, Cannavale R, Vallelonga T, Garagiola U, Merlo A. Improvement of masticatory kinematic parameters after correction of unilateral posterior crossbite: Reasons for functional retention. Angle Orthod. 2017 Nov;87(6):871-877. doi: 10.2319/020917-98.1. Epub 2017 Aug 3. PMID: 28771046; PMCID: PMC8317566.
38. Bode C, Ghaltakhchyan N, Silva ER, Turvey T, Blakey G, White R, Mielke J, Zajac D, Jacox L. Impacts of Development, Dentofacial Disharmony, and Its Surgical Correction on Speech: A Narrative Review for Dental Professionals. Appl Sci (Basel). 2023 May;13(9):5496. doi: 10.3390/app13095496. Epub 2023 Apr 28. PMID: 37323873; PMCID: PMC10270670.
39. Nosetti L, Zaffanello M, De Bernardi di Valserra F, Simoncini D, Beretta G, Guacci P, Piacentini G, Agosti M. Exploring the Intricate Links between Adenotonsillar Hypertrophy, Mouth Breathing, and Craniofacial Development in Children with Sleep-Disordered Breathing: Unraveling the Vicious Cycle. Children (Basel). 2023 Aug 21;10(8):1426. doi: 10.3390/children10081426. PMID: 37628425; PMCID: PMC10453215.
40. nada E, Saitoh I, Kaihara Y, Yamasaki Y. Factors related to mouth-breathing syndrome and the influence of an incompetent lip seal on facial soft tissue form in children. Pediatric Dental Journal.2021;31(1):1-10.
41. Xi J, Si XA, Kim J, Zhang Y, Jacob RE, Kabilan S, Corley RA. Anatomical Details of the Rabbit Nasal Passages and Their Implications in Breathing, Air Conditioning, and Olfaction. Anat Rec (Hoboken). 2016 Jul;299(7):853-68. doi: 10.1002/ar.23367. Epub 2016 May 17. PMID: 27145450; PMCID: PMC4907833.
42. Pacheco MC, Casagrande CF, Teixeira LP, Finck NS, de Araújo MT. Guidelines proposal for clinical recognition of mouth breathing children. Dental Press J Orthod. 2015 Jul-Aug;20(4):39-44. doi: 10.1590/2176-9451.20.4.039-044.oar. PMID: 26352843; PMCID: PMC4593528.
43. Ferrés-Amat E, Pastor-Vera T, Ferrés-Amat E, Mareque-Bueno J, Prats-Armengol J, Ferrés-Padró E. Multidisciplinary management of ankyloglossia in childhood. Treatment of 101 cases. A protocol. Med Oral Patol Oral Cir Bucal. 2016 Jan 1;21(1):e39-47. doi: 10.4317/medoral.20736. PMID: 26595832; PMCID: PMC4765751.
44. Liu Y, Zhou JR, Xie SQ, Yang X, Chen JL. The Effects of Orofacial Myofunctional Therapy on Children with OSAHS's Craniomaxillofacial Growth: A Systematic Review. Children (Basel). 2023 Mar 31;10(4):670. doi: 10.3390/children10040670. PMID: 37189919; PMCID: PMC10136844.
45. Martos-Cobo E, Mayoral-Sanz P, Expósito-Delgado AJ, Durán-Cantolla J. Effect of rapid maxillary expansion on the apnoea-hypopnoea index during sleep in children. Systematic review. J Clin Exp Dent. 2022 Sep 1;14(9):e769-e775. doi: 10.4317/jced.59750. PMID: 36158770; PMCID: PMC9498642.
46. Ellis LA, Blakely B, Hazell P, Woolfenden S, Hiscock H, Sarkozy V, Gould B, Hibbert PD, Arnolda G, Ting HP, Wiles LK, Molloy CJ, Churruca K, Warwick M, Braithwaite J; CareTrack Kids Investigative Team. Guideline adherence in the management of attention deficit hyperactivity disorder in children: An audit of selected medical records in three Australian states. PLoS One. 2021 Feb 8;16(2):e0245916. doi: 10.1371/journal.pone.0245916. PMID: 33556083; PMCID: PMC7869992.
47. Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, Evans SW, Flinn SK, Froehlich T, Frost J, Holbrook JR, Lehmann CU, Lessin HR, Okechukwu K, Pierce KL, Winner JD, Zurhellen W; SUBCOMMITTEE ON CHILDREN AND ADOLESCENTS WITH ATTENTION-DEFICIT/HYPERACTIVE DISORDER. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019 Oct;144(4):e20192528. doi: 10.1542/peds.2019-2528. Erratum in: Pediatrics. 2020 Mar;145(3): PMID: 31570648; PMCID: PMC7067282.
48. Andersen IG, Holm JC, Homøe P. Obstructive sleep apnea in children and adolescents with and without obesity. Eur Arch Otorhinolaryngol. 2019 Mar;276(3):871-878. doi: 10.1007/s00405-019-05290-2. Epub 2019 Jan 28. PMID: 30689039.
49. Carneiro-Barrera A, Díaz-Román A, Guillén-Riquelme A, Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obes Rev. 2019 May;20(5):750-762. doi: 10.1111/obr.12824. Epub 2019 Jan 4. PMID: 30609450.
50. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as a treatment for obstructive sleep apnoea. Sleep Med Rev. 2000 Oct;4(5):435-52. doi: 10.1053/smrv.2000.0114. PMID: 17210276.
51. Sinha R, Raut S. Management of nocturnal enuresis - myths and facts. World J Nephrol. 2016 Jul 6;5(4):328-38. doi: 10.5527/wjn.v5.i4.328. PMID: 27458562; PMCID: PMC4936340.
52. Wang Q, Jia P, Anderson NK, Wang L, Lin J. Changes of pharyngeal airway size and hyoid bone position following orthodontic treatment of Class I bimaxillary protrusion. Angle Orthod. 2012 Jan;82(1):115-21. doi: 10.2319/011011-13.1. Epub 2011 Jul 27. PMID: 21793712; PMCID: PMC8881045.
53. Alqahtani ND, Alshammari R, Almoammar K, Almosa N, Almahdy A, Albarakati SF. Post-orthodontic cephalometric variations in bimaxillary protrusion cases managed by premolar extraction - A retrospective study. Niger J Clin Pract. 2019 Nov;22(11):1530-1538. doi: 10.4103/njcp.njcp_125_19. PMID: 31719274.