You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Treatment of the ailing/failing implant can be a challenging endeavor for dentists. Periodically, continuing education courses arise that attempt to make this process fairly simple and predictable. However, treating threaded implants that are contaminated or that have granulation and purulence surrounding them is difficult and results in a guarded prognosis at best. Because modern implants have become more cost-effective, oftentimes, practitioners will simply choose to remove an implant that is demonstrating significant bone loss rather than allow the negative process to continue with failure inevitable. Bone loss that occurs around dental implants should not be ignored and should be treated aggressively when possible. Following extraction of a failed implant, the residual bone at the site may be grafted and allowed to heal, or a new wider or longer implant may be immediately placed. However, the cost of removal and replacement may be prohibitive to patients who assumed that the implant would "last a lifetime."
Problems with dental implants may arise for a variety of reasons and can lead to horizontal or vertical bone loss. Poor oral hygiene can result in bacterial invasion, inflammation, and bone loss.1 In some circumstances, the dental implant may fail to integrate due to overheating of the bone during preparation of the surgical site, overtorquing of the implant during placement, contamination of the implant surface, improper positioning of the implant resulting in inadequate initial stability, or healing issues related to the patient. Smoking may affect the initial integration process because the smoke, tar, and heat generated reduces cell formation. In addition, forces placed on the implant may result in micromovement and failure to integrate, and obstructive occlusal forces may result in horizontal bone changes.1
Implant Failure and Treatment
Late implant failure can be described as the loss of hard-tissue structure around a dental implant over time after initial integration. Peri-implantitis is a significant cause of late implant failure2; therefore, maintaining periodontal health is not only critical to the overall health of the patient but also an important consideration in achieving a positive long-term prognosis for the implant and the prosthetic reconstruction. Minimizing bacterial count while maintaining occlusal harmony is an effective method of keeping implant restorations viable. According to the results of a literature review, peri-implantitis is prevalent in approximately 10% of implants and 20% of implant patients.3 In a study that evaluated patients who had received implants 9 years previously, 45% presented with peri-implantitis (bleeding on probing, suppuration, and bone loss greater than 0.5 mm). Moderate/severe peri-implantitis (bleeding on probing, suppuration, and bone loss greater than 2 mm) was diagnosed in 14.5%."4
Several treatments are attempted to maintain the function of these implants, and sometimes, invasive surgical procedures are also attempted. Regardless of the method chosen, the implant surface must be decontaminated for hard- and soft-tissue healing to occur. Regarding exposed diseased sites, several approaches to decontamination have been discussed in the literature, including mechanical debridement and smoothing of the implant surface, the use of chemotherapeutic agents, and the provision of laser therapy.5 Curettes, ultrasonic scalers, and air abrasion units are sometimes used as well as strong pharmaceutical agents, such as chlorhexidine digluconate and tetracycline.5 The problem with these methods is that limited access to the area, the presence of resistant bacteria, and increased bacterial attachment associated with the threaded surface of the implant body can make cleaning the area challenging. Alternatively, lasers can get to surfaces that mechanical techniques cannot.
Dental Lasers
There are many different varieties of lasers that are used in periodontal correction, including diode, neodymium-doped yttrium aluminum garnet (Nd:YAG), erbium-doped yttrium aluminum garnet (Er:YAG), and carbon dioxide (CO2) lasers. These laser types can be used to disinfect the titanium implant surface and aid in healing because they are designed to specifically target certain substances, which are known as chromophores. The primary chromophores of dental lasers include water, hemoglobin, melanin, and hydroxyapatite.6
CO2 lasers are used for rapid, shallow soft-tissue removal and achieving hemostasis. Similarly, the Nd:YAG laser is also used to coagulate and incise tissue and provides good hemostasis. It has been used for sulcular debridement in areas of the mouth that exhibit periodontal disease. Erbium lasers, which have two significant wavelengths, have a high affinity for hydroxyapatite and are the lasers of choice for hard-tissue procedures. And finally, the diode laser is used to contour soft tissue; uncover buried, integrated dental implants; remove inflamed tissue; and perform photostimulation.6,7
Photobiomodulation therapy is a treatment that uses a form of nonthermal, low-level laser light energy to penetrate the tissue with the goal of stimulating photophysical and photochemical changes in the cells. This potentially results in improved wound healing and tissue regeneration by increasing the blood circulation to the surgical site and reducing the inflammation caused by infection through the vasodilation of small arteries and the lymph system. In addition, photobiomodulation therapy may help to promote osseointegration, and positive effects have been observed in osteoclast and osteocyte action.8-11
Diode Laser Decontamination
Lasers vaporize diseased tissue based on their wavelength, power setting, and duration of use. Diode lasers are designed to selectively target dark, necrotic tissue while preserving healthy tissue and will not cut bone.6 They work by transmitting energy that is absorbed by dark pigmented tissue, hemoglobin, or melanin. A carbon-initiated tip concentrates the energy, and a photothermal reaction results. The tissue is heated to a range of 100°C to 150°C, and localized vaporization occurs around the zone of carbonization.12,13
Numerous studies have been published showing the efficacy of diode lasers in reducing long-term bacterial growth.13,14 Specifically, the diode laser creates a bactericidal effect by increasing temperature, which also promotes osteoblastic and fibroblastic activity.9,13,15The tissue ablation and bacterial reduction results in tissue remodeling and increased bone metabolism and may help in the regeneration of hard tissue around a defective titanium implant.14 Importantly, the diode laser does not damage the titanium surface of implants.16-18The long, thin glass fiber tip is used to sterilize the contaminated surface, and the biostimulation of mesenchymal stem cells aids in the integration of new bone. Light stimulated emission is reflected, scattered, or absorbed by the surrounding tissue.12,19-21
In a study in which patients with peri-implantitis affecting single tooth implants were treated using laser decontamination, implantoplasty surgery, or a combination of both, after a 9-year period, the treatments achieved success rates ranging from 83% to 92%.2 Long-term results of such treatments are challenging to identify in the literature, but decontamination of the inflamed area around a compromised implant seems to increase the chances of success.6,2 Although inflammation and bone loss around dental implants can occur for many reasons, the goal of the treatments discussed here is to stabilize hard-tissue shrinkage in the hope of maintaining a functional prosthesis without the need for complete removal of the fixture and re-treatment. Eliminating the source of the infection increases the viability of a positive prognosis.
In the following case report, the type of bone dehiscence discussed could have been caused by improper angulation of the implant.1 Ideally, 1 mm to 2 mm of facial and palatal bone should surround an implant to provide for proper osseointegration.22
Case Report
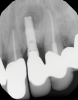
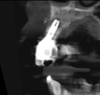
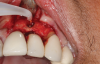
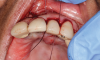
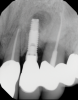
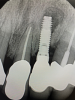
In this case, a dental implant that had been placed 10 years prior was functional, stable, and esthetically acceptable to the patient. However, a significant fistula was present on the facial-apical aspect of the ridge in the maxillary lateral incisor area. This area was painfully sensitive to touch and demonstrated purulence when squeezed. To evaluate the lesion, first, a conventional digital radiograph was acquired, which revealed an apical radiolucency at the apex of the implant (Figure 1). Further analysis using cone-beam computed tomography (CBCT) demonstrated a fistula from that site to the oral environment (Figure 2). Treatment options were discussed, including removal of the implant, followed by grafting, a healing period, and replacement of the implant and implant-retained crown. If this option was selected, a transitional appliance would need to be created. Another option was to attempt to salvage the implant and implant crown by treating the infection and grafting the site to create a new boney wall and eliminate the fistula. Ultimately, the patient accepted this option to attempt to salvage the fixture and crown.
Peri-implantitis was not the culprit behind the creation of this infected site; the lesion developed as a result of the position of the implant. Although the implant was initially stable upon placement, it was angled such that its apex had penetrated through the facial plate of bone. Without a sagittal CBCT analysis or a releasing flap performed to evaluate the initial placement, the surgeon could easily miss this. Regardless, the circumstances warranted evaluation of the apical aspect of the implant to attempt to salvage it.
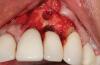
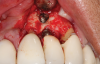
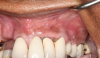
Following infiltration of the facial attached gingiva and palatal area with articaine hydrochloride 4% and epinephrine 1:100,000 (Septocaine®, Septodont [alternatively: Articadent®, Dentsply Sirona; Orabloc®, Pierrel]), an envelope releasing incision was made extending one full tooth over. To prevent trauma to the mucosal tissue, vertical incisions were not made (Figure 3). Incising the mucosa results in the release of prostaglandins and histamine, which increases postoperative discomfort. For this patient, staying in attached gingiva provided excellent control of the flap and allowed clear access to the apical lesion.
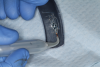
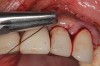
This is where the treatment became challenging. First, a curette was used to attempt to remove the granulation tissue from around the apex of the implant circumferentially. This was a difficult endeavor, especially on the palatal aspect of the site. Following curettage, an 810 nm diode laser (NV® PRO3, DenMat [alternatively: Picasso+™, AMD Lasers; Gemini™, Ultradent]) was used to treat the apical portion of the implant. After the laser tip was initiated using dark occlusal paper (Figure 4), it was used to debride the remaining necrotic tissue and decontaminate the entire site, reducing the bacterial count (Figure 5 and Figure 6).
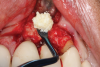
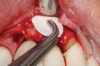
There are many grafting materials available today, including allografts (from a human source), xenografts (from another species), and alloplasts (from synthetic materials).23 Following debridement and sterilization of the apical lesion, the site was grafted with a mineralized cortical/cancellous bone allograft material (Newport Biologics™ Mineralized Cortico/Cancellous Allograft Blend, Glidewell Direct [alternatively: OSSIF-I sem™ Mineralized Cortical/Cancellous Bone Allograft, Surgical Esthetics; enCore® 50/50 Cortical & Cancellous Allograft, Osteogenics]). The material was packed firmly around the entire implant body but not crushed (Figure 7). Next, a resorbable collagen membrane (Newport Biologics™ Resorbable Collagen Membrane 3-4, Glidewell Direct [alternatively: Bio-Gide®, Geistlich Biomaterials; BioMend®, Zimmer Biomet]) was trimmed to extend approximately 2-mm beyond the borders of the defect and then passively positioned to serve as a barrier against epithelial growth during the healing and remodeling phase (Figure 8). A sling suture technique using polyglactin 910 suture was then used to reposition the envelope reflection (Figure 9 through Figure 11). Polyglactin 910 suture is a smooth, synthetic, absorbable, braided suture made of polyglycolic acid that is broken down over time by hydrolysis. This suture material reduces the inflammatory response that can be observed with other suture materials.
Although grafting can be a challenging process, when it is properly completed, it helps support the soft-tissue structure and prevent further periodontal failure.23 Primary stability is imperative for the long-term success of any implant. Defect grafting procedures allow for the regeneration of bone, and a proper protocol is an essential part of the implant dentist's skill set.
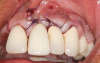
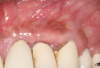
In this case, the procedure was precise, and the patient experienced minimal postoperative discomfort. An immediate postoperative digital radiograph was acquired to evaluate the radiolucent appearance of the grafted apical defect (Figure 12). After 8 weeks, the tissue appeared to be healing quickly and predictably (Figure 13), and a follow-up sagittal CBCT analysis demonstrated a new wall forming where the dehiscence had been present (Figure 14). The patient was recalled again after 5 months for radiographic analysis and intraoral evaluation, which revealed that the healing was progressing without complication (Figure 15 through Figure 18).
Conclusion
Dental implants have proven to be an outstanding treatment modality for the replacement of missing teeth. Their design facilitates outcomes that are both functional and esthetic. However, problems can arise that may compromise the long-term prognosis of implant therapy that can be both frustrating and challenging to the dental practitioner. Bone loss, the result of periodontal disease, can affect stability. Treatment of an apical dehiscence around a dental implant may seem complicated, and the task of debriding such an area where the implant threads are exposed can be daunting. The more implant surface area that is exposed to diseased tissue, the more difficult it is to treat and the greater the likelihood of implant loss. As long as the implant is stable, and there is an adequate band of healthy attached gingiva at its cervical aspect, attempting to salvage the implant is a reasonable alternative to total implant removal, grafting, and eventual replacement of the fixture. Lasers provide a bactericidal effect during debridement. The treatment ablation targets only the undesirable tissue and does not damage the adjacent healthy tissue. Therefore, wound healing is more profound because the inflammatory response is reduced. While enzymatic inhibitors are promoted, and the infection is virtually eliminated, stimulation of the hard and soft tissue occurs to promote complete wound healing.
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
References
1. Froum J, Rosen P, Wang HL. Why do implants fail? Compend Contin Educ Dent. 2017;38(6):360.
2. Pommer B, Haas R, Mailath-Pokorny G, et al. Periimplantitis treatment: long-term comparison of laser decontamination and implantoplasty surgery. Implant Dent. 2016;25(5):646-649.
3. Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(suppl 6):67-76.
4. Derks J, Schaller D, Håkansson J, et al. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95(1):43-49.
5. Mellado-Valero A, Buitrago-Vera P, Sola-Ruiz M, Ferrer-Garcia J. Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal. 2013;18(6):e869-e876.
6. Verma SK, Maheshwari S, Singh RK, Chaudhari PK. Laser in dentistry: an innovative tool in modern dental practice. Natl J Maxillofac Surg. 2012;3(2):124-132.
7. Romanos GE, Gupta B, Yunker M, et al. Lasers use in dental implantology. Implant Dent. 2013;22(3):282-288.
8. Gholami L, Asefi S, Hooshyarfard A, et al. Photobiomodulation in periodontology and implant dentistry: part 1. Photobiomodul Photomed Laser Surg. 2019;37(12):739-765.
9. Ohsugi Y, Niimi H, Shimohira T, et al. In vitro cytological responses against laser photobiomodulation for periodontal regeneration. Int J Mol Sci. 2020;21(23):9002.
10. Gholami L, Asefi S, Hooshyarfard A, et al. Photobiomodulation in periodontology and implant dentistry: part 2. Photobiomodul Photomed Laser Surg. 2019;37(12):766-783.
11. Tang E, Arany P. Photobiomodulation and implants: implications for dentistry. J Periodontal Implant Sci. 2013;43(6):262-268.
12. Deppe H, Horch H. Laser applications in oral surgery and implant dentistry. Lasers Med Sci. 2007;22(4):217-221.
13. Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217-269.
14. Kurtzman GM, Weitz M, Kaminer R, Gober DD. Treatment of peri-implantitis with a diode laser: a long-term case follow-up. Inside Dentistry. 2016;12(11):54-58.
15. Ashnagar S, Nowzari H, Nokhbatolfoghahaei H, et al. Laser treatment of peri-implantitis: a literature review. J Lasers Med Sci. 2014;5(4):153-162.
16. Natto ZS, Aladmawy M, Levi PA, Wang HL. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2015;30(2):338-345.
17. Trivedi AR, Patel V, Gupta S, Jathal BS. Treatment of peri-implantitis with implantoplasty and diode laser. Int J Clin Med Images. 2019;6(7):658-660.
18. Crispino A, Figliuzzi MM, Iovane C, et al. Effectiveness of a diode laser in addition to non-surgical periodontal therapy: study of intervention. Ann Stomatol (Roma). 2015;6(1):15-20.
19. Winter RB. Practical laser applications in general practice. Dent Today. 2017;36(6):78,80,82-83.
20. Barboza C, Ginani F, Soares D, et al. Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (Sao Paulo). 2014;12(1):75-81.
21. Giannelli M, Chellini F, Sassoli C, et al. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: effects and mechanisms of action. 2013;228(1)172-181.
22. Belser UC, Bernard JP, Buser D. Implants in the esthetic zone. In: Lindhe J, Lang NP, Karring T, eds. Clinical Periodontology and Implant Dentistry. Oxford, UK: Blackwell Munksgaard; 2008:1146-1174.
23. Kumar P, Vinitha B, Fathima G. Bone grafts in dentistry. J Pharm Bioallied Sci. 2013;5(suppl 1):s125-s127.