You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT), also referred to as "stem cell" or "bone marrow" transplantation, is a potentially life-saving procedure for individuals with malignant hematological (blood-related) diseases, such as leukemia and lymphoma, as well as non-malignant conditions, such as bone marrow failure syndromes and hemoglobinopathies.1 According to the Center for International Blood and Marrow Transplant Research,2 over 9,000 alloHCT procedures were performed in the United States in 2018, and this number has been steadily increasing.3 Recent developments in technology and supportive care practices have led to improved post-transplant survivorship.4-6 While this progress is highly encouraging, survivors are at risk for a range of post-transplant complications, including chronic graft-versus-host disease (cGVHD), a relatively common and potentially serious condition affecting a range of organs, frequently including the oral cavity.7,8 Appropriate screening and management of post-transplant complications require a multidisciplinary team approach, in which oral health care providers play a central role.9 This review provides an overview of alloHCT with a focus on oral cGVHD and the role of the dental hygienist in the management of this complex condition.
Allogeneic Hematopoietic Cell Transplantation (alloHCT)
AlloHCT is a non-surgical therapeutic procedure, similar to a blood transfusion, in which a patient receives healthy hematopoietic cells from a related or unrelated donor via a central intravenous catheter.10,11 Prior to transplantation, the patient undergoes a conditioning regimen, during which residual cancer cells (in the case of hematologic malignancies) are targeted for destruction and the patient is immunosuppressed via chemotherapy, total body irradiation, and/or immunotherapeutic agents.10,11 The conditioning regimen is essential for preventing graft rejection and for allowing the donor stem cells to establish hematopoiesis.10,11 The donor cells ultimately produce a new functional bone marrow capable of producing healthy red blood cells, white blood cells, and platelets.10,11During this time, the patient's laboratory values are closely monitored, particularly the complete blood count (CBC) with differential. The patient is said to be "engrafted" when the absolute neutrophil count is greater than 500 cells/uL on three consecutive days, typically by day +30 after alloHCT. 10,11 Following engraftment, patients continue to be monitored closely due to risk for disease relapse, infection, and other transplant-associated complications, such as graft-versus-host disease (GVHD).10 To reduce the risk of developing GVHD, all patients receive GVHD prophylaxis post-alloHCT, which typically consists of a short course of methotrexate and a longer course of a calcineurin inhibitor.12
There are many oral health considerations to assess immediately before, during, and following alloHCT. A comprehensive oral evaluation should be completed prior to transplantation and a dental clearance should be obtained as the recommended standard of care to reduce the risk of bacteremia and morbidity post-alloHCT.13 At this time, any urgent dental needs should be addressed, including any extractions, periodontal therapy, and the elimination of local trauma. Ideally this treatment should be completed two weeks before transplantation to allow sufficient post-operative healing of oral tissues.13 During and after alloHCT, patients have lower white blood cell counts, making them more susceptible to oral herpetic and opportunistic infections caused by Candida. Thus, patients who are seropositive for herpes simplex virus should receive acyclovir prophylaxis to prevent viral reactivation, and most medical centers use antifungal prophylaxis to prevent oral candidiasis.13 Furthermore, the conditioning and GVHD prophylaxis regimens are associated with the risk of oral mucositis, a painful ulcerative condition that can limit one's ability to eat, drink and speak.14 While mild oral mucosal pain may be addressed with topical anesthetics and analgesics, more debilitating pain may necessitate opioids, and total parenteral nutrition may be indicated if oral intake is severely limited.13,15
The overall one- and five-year survival rates post-alloHCT are approximately 70% and 55%, respectively.16The leading causes of mortality are cancer recurrence and complications related to GVHD, including deaths due to infection and immunosuppressive treatment.6 Consequently, long-term follow-up care is critical for reducing the risk of complications related to the transplant.9,17
Chronic Graft-Versus-Host-Disease (cGVHD)
Graft-Versus-Host-Disease is a complex immune-mediated disease resulting from an incompatibility between the donor (graft) and patient (host) cells. It is classified as either acute (aGVHD) or chronic (cGVHD) based on differentiating clinical and pathologic features. Acute GVHD typically occurs within the first 100 days following alloHCT and cGVHD usually develops after day +100; however, these time points are somewhat arbitrary.10 Acute GVHD most commonly affects the skin, liver, and gastrointestinal (GI) tract.18 Chronic GVHD most commonly affects the skin, oral cavity, eyes, GI tract, liver, and lungs.9,19 Acute and chronic features may overlap, yet cGVHD has distinct characteristics affecting the oral cavity. Signs and symptoms of cGVHD are similar to those of many autoimmune conditions and can profoundly affect systemic health and one's overall quality of life.9,20 Table I provides a summary of chronic GVHD clinical features.
Chronic GVHD affects up to 50% of alloHCT recipients and often follows aGVHD, but it can also develop de novo (without prior aGVHD) and may present upon tapering of GVHD prophylaxis (e.g., calcineurin inhibitors, such as cyclosporine or tacrolimus).18 Additional risk factors include the use of peripheral blood stem cells (versus bone marrow) as the graft source, unrelated donors (versus related donors, such as siblings), human leukocyte antigen (HLA) mismatching between donor and recipient, female donor to male recipient, older donor age, and history of donor lymphocyte infusion (a therapy used in patients with disease relapse).19,21 While the incidence of cGVHD is lower in pediatric alloHCT recipients (<18 years old), clinical manifestations observed in this population are similar to those seen in adults.22
The pathophysiology of cGVHD is highly complex and involves multiple biological processes, including immune dysregulation, chronic inflammation, and fibrosis.23 Histo-compatibility differences between donor and recipient HLA gene products cause the donor T-cells to recognize the host HLA antigens as "foreign," which triggers an attack on the healthy host tissues.24-26 This inflammatory response can impact any organ system in the body and cause tissue fibrosis, varying degrees of tissue damage, and functional impairment.27
Oral Manifestations of cGVHD
Following the skin, the oral cavity is the second most common site affected by cGVHD, with up to 70% of patients presenting with oral features.28,29 Oral cGVHD is typically diagnosed by an oncologist or oral medicine specialist based on a thorough health history assessment and clinical examination; in some cases, a biopsy may be required to support the diagnosis or rule out other conditions. Oral cGVHD can affect the lips, oral mucosa, and salivary glands. Clinical features may include lichen planus-like manifestations, salivary gland hypofunction, and orofacial fibrosis.30,31 Oral cGVHD can cause mucosal pain and sensitivity, xerostomia, and indirect effects, such as altered diet, compromised ability to maintain good oral hygiene, and, thus, increased risk for dental caries and gingival disease.13
Oral mucosal lesions
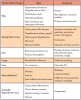
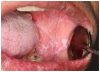
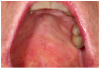
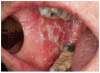
Mucosal lesions are characterized by three main signs: 1) lichenoid inflammation, 2) erythema, and 3) ulcerations.20 Lichenoid inflammation appears as white reticular streaks or lacey lines that resemble Wickham striae observed in oral lichen planus and are considered to be a diagnostic feature of oral cGVHD7,30 (Figure 1a). While these lesions may occur anywhere in the oral cavity, they most frequently appear on the buccal mucosa and tongue.31,32 Lichenoid lesions may be accompanied by varying degrees of erythema and ulceration, which are features often associated with more severe symptoms (Figure 1b-c). Ulcerations represent a breakdown in oral mucosa and can be particularly symptomatic, limiting functions such as oral nutrition, speech, and oral hygiene maintenance.33
Pain at rest may be reported; however, the hallmark symptom of mucosal inflammation is sensitivity to spicy, acidic, hard, and crunchy foods.31,34 Toothpaste containing sodium lauryl sulfate and strong flavoring agents (e.g., mint and cinnamon) may also be intolerable.35 While symptoms are generally worse with more severe clinical features, it is possible for a patient with relatively mild lichenoid changes to experience symptoms similar to or worse than those of a patient presenting with erythema and ulcerations.31
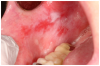
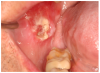
Superficial recurrent mucoceles are also common in patients with oral cGVHD. They appear as transient, saliva-filled, raised lesions, secondary to inflammation of minor salivary glands, and are most commonly located on the hard and soft palate or labial mucosa30 (Figure 1d). While these lesions are generally asymptomatic, they may be a nuisance or a source of concern to the patient. Treatment other than recognition and patient reassurance is rarely indicated.36
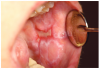
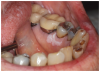
Furthermore, patients with oral cGVHD are at increased risk for developing oral squamous cell carcinoma37,38 (Figure 2a). This may be due to prolonged mucosal inflammation, immune dysregulation, and iatrogenic immunosuppression.39 Oral squamous cell carcinoma may arise from areas of oral leukoplakia, which generally presents distinctly from the white reticular features of mucosal cGVHD (Figure 2b).
Salivary gland dysfunction
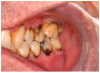
Salivary gland dysfunction and xerostomia associated with cGVHD mimic the clinical features and symptoms of Sjögren syndrome. Hyposalivation impairs the protective activity of saliva, elevating the risk for dental caries and accelerating the progression of white spot lesions, and in some cases, to rampant dental caries and subsequent tooth loss31,40,41 (Figure 2c). Hyposalivation also reduces oral lubrication, which can lead to difficulty speaking, eating, and dysphagia.42 Furthermore, the reduction of salivary proteins (e.g., histatin, lactoferrin, calprotectin) can diminish antimicrobial and antifungal activity, thereby increasing the risk for recurrent oral candidiasis.30,31,43 Oral candidiasis most frequently presents as white pseudomembranous patches but may also present with diffuse erythema (Figure 2d).
Orofacial sclerosis
Although relatively infrequent, sclerosis of the perioral skin and intraoral mucosal tissues may occur and can be associated with significant morbidity.30,44 Sclerodermatous cutaneous disease, a chronic hardening and tightening of the skin and connective tissues, can extend to the facial and perioral tissues, leading to impaired mouth opening and trismus.45 In some cases, involvement of muscles can lead to transient painful myospasms, which can also contribute to trismus. These conditions can compromise the patient's ability to perform oral self-care and can complicate the provision of professional dental care.
Management of Oral cGVHD
Many patients with oral cGVHD will be managed with systemic medications due to cGVHD activity in other organ systems.36 Systemic therapy may or may not adequately control oral cGVHD, as an oral response is highly variable. Furthermore, it is not uncommon for signs and symptoms of oral cGVHD to persist even after systemic therapy resolves cGVHD manifestations in other organ systems.30,36
Dental hygienists, as part of a multi-disciplinary care team, play an important role in the management of patients with oral cGVHD.46 Patients will typically return to routine dental care approximately one year following alloHCT.9 At that time, the assessments should include a thorough review of medical history and medications, as well as a comprehensive extraoral and intraoral examination to identify clinical signs and symptoms of systemic and oral diseases.
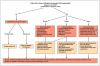
The extraoral examination includes a visual inspection of the skin and lips, careful palpation of the submandibular and cervical lymph nodes, and assessment of temporomandibular mandibular joint (TMJ) function and mouth opening. The intraoral examination thoroughly evaluates all mucosal tissues, including the soft palate and tonsillar pillars. It can be challenging to distinguish between suspicious abnormalities and manifestations associated with oral cGVHD, but certain features that should be of particular concern as part of the oral cancer screening include atypical white plaques, focal masses, tissue induration, and non-healing and necrotic ulcers.31 Obtaining periodic intraoral photographs of mucosal findings is helpful for documentation and assessment of changes over time; suspicious abnormalities should be referred for biopsy.31 Risk factors for oral cancer, such as tobacco use and excessive alcohol intake should be assessed, and patients should be counseled accordingly.47 The dental hygiene care plan should address all symptoms of oral cGVHD, with a focus on preventing sequelae of disease, such as hyposalivation-induced dental caries and oral candidiasis. Figure 3 outlines the process of care for a patient with a history of alloHCT, and the oral health considerations and management recommendations are summarized in Table II.
Oral mucosal lesion management
When caring for patients with oral cGVHD, the overall goal is to manage symptoms rather than explicitly resolve or heal lesions.31 Oral mucosal symptoms are managed with high potency topical corticosteroids, generally in the form of a solution or gel.34 Topical tacrolimus, a non-steroid immunomodulatory agent, can also help to manage symptoms and is commercially available in the form of a 0.1% ointment or can be compounded as a solution.48 Solutions are swished for 4-6 minutes then expectorated, and are beneficial for treating extensively involved and hard-to-reach areas.48 In addition to solution-based therapy, gels may be applied focally to symptomatic lesions where the disease is more localized or more intensive treatment is needed. Gels can be delivered via gauze or an occlusive custom tray (e.g., for gingival or palatal involvement) from one to four or more times daily, depending on the degree of symptoms and level of response.31 With improvement or resolution of symptoms, therapy is often tapered or discontinued but can be resumed or intensified if symptoms flare. Bland oral rinses (e.g., 0.9% saline) or "magic mouthwashes" containing a topical anesthetic and antihistamine can also be prescribed to help reduce oral mucosal pain.15 Topical tacrolimus is preferred when treating lesions of the lip vermillion due to the potential for irreversible atrophy and thinning of the tissue with topical steroid therapy.31 Topical anesthetics should be considered for in-office use during dental visits for pain control, as needed. In addition, lip care should include adequate moisturization and sun protection (i.e., SPF 30+) given the increased risk for skin cancers post-transplant.36
Secondary candidiasis is a common complication associated with the use of topical steroids in the oral cavity.49 Risk factors include systemic immunosuppression, topical corticosteroid therapy, and salivary gland dysfunction.30 Diagnosis is usually based on clinical examination, although features may be difficult to distinguish from cGVHD.49 Fluconazole is the most common systemic antifungal medication used to treat or prevent oral candidiasis but must be used with caution in patients on systemic therapy due to potential drug interactions.50 Clotrimazole troches and nystatin suspensions are topical antifungal medications that should be used with caution due to their sugar content and cariogenic properties.15 Sugar-free versions of these drugs are available and can be requested when prescribed.
Angular cheilitis may also be present, for which antifungal creams or ointments can be prescribed.28,31 Patients wearing removable dentures and appliances should be advised to remove, soak, and brush their dentures/appliances daily with a commercial cleanser and denture brush to reduce their risk of oral yeast infections. Individuals with fungal infections should treat the denture/appliance with an anti-fungal remedy, such as chlorhexidine, nystatin, or dilute bleach solution (1:10) to avoid reinfection from the oral prosthesis. Those who experience recurrent infections may benefit from long-term antifungal prophylaxis.15
Salivary gland hypofunction and dental caries risk considerations
The symptoms of salivary hypofunction may be managed with over-the-counter products for dry mouth in the form of rinses, gels, sprays, and saliva substitutes.48 In addition to ensuring good hydration with frequent water intake, the use of sugar-free gum and lozenges can stimulate salivary flow, and bland rinses (e.g., 0.9% normal saline or 0.5% sodium bicarbonate rinses) may ease the discomfort of xerostomia.36 Salivary flow can also be improved with prescription sialagogue medications (e.g., pilocarpine and cevimeline).51-53 Prior to prescribing sialagogue therapy, clinicians should ensure that there are no medical contraindications (e.g., narrow angle glaucoma), and possible side effects (e.g., sweating) should be reviewed with the patient and their oncology care provider(s).
When patients with alloHCT return to the dental office for routine follow-up care, their caries risk assessment and dental hygiene care plan should be updated based on their current health status. The three primary conditions associated with oral cGVHD (oral mucosal lesions, salivary hypofunction, and orofacial sclerosis) compound the risk for dental caries, potentially accelerating disease progression. Oral mucosal pain and sensitivity may lead to difficulty in performing oral self-care, as well as a shift to a softer diet that requires less mastication and often contains higher levels of fermentable carbohydrates.54,55 Additionally, reduced quantity and quality of saliva inhibit oral cleansing ability, antimicrobial activity, neutralization of acids, and tooth remineralization.56 Patients with limited mouth opening may also encounter challenges with performing oral self-care, and patients experiencing other comorbidities may suffer from disease management fatigue, contributing to suboptimal homecare.52
Dental hygienists can work with patients to help tailor their oral self-care routines. Brushing may be best tolerated with a non-mint flavored, fluoridated toothpaste and an extra soft bristle toothbrush.36 Interdental cleaning can be made easier for patients with restricted mouth opening via the use of floss holders, floss picks, interdental brushes, and oral irrigators. For those presenting with moderate to extreme dental caries risk, a 5,000 ppm (1.1%) sodium fluoride toothpaste should be prescribed for twice-daily use.57,58 Patients presenting with high caries risk may also benefit from the application of prescription-strength fluoride gel via trays for 5 minutes daily. In-office fluoride varnish application every 3 or 6 months is recommended for patients with high or moderate risk for dental caries, respectively.57,59 Silver diamine fluoride is another caries-preventive and caries-arresting agent that can be applied in a site-specific manner to slow or arrest the dental caries process.60
A dietary assessment should also be performed, and patients should receive nutritional counseling to help minimize their caries risk. Patients should be advised to avoid cariogenic foods and drinks, including sugar-sweetened beverages, gums, and lozenges while increasing their intake of non-cariogenic and cariostatic foods.61,62 The importance of twice-yearly or more frequent dental examinations and dental hygiene recare visits must be emphasized in coordination with the patient's primary medical team.31 Patients will benefit from individualized and detailed written instructions for all oral self-care recommendations.
Orofacial sclerosis considerations
Sclerodermatous oral cGVHD may be managed with long-term physical therapy to improve or at least maintain stable mouth opening.31,63,64 Dental hygiene care appointments may be challenging for the patient and provider alike due to the patient's limited opening. Adaptations may be necessary to increase patient comfort and acceptance of care. These may include the use of bite blocks, shorter appointments, and frequent breaks during longer appointments.64 When mouth opening is limited, dental hygienists may need to assist patients in identifying oral physiotherapy aids that improve access and effectiveness (e.g., an oral irrigator).65
Conclusion
Patients with oral cGVHD present with unique challenges that require special attention during dental hygiene care. While this is a relatively small subset of the general population, the number of people surviving long-term after alloHCT is growing and is expected to continue to increase over time. Although major clinical features of oral cGVHD are not directly treated in the dental setting, the dental hygienist plays a central role in detecting, assessing, documenting, and educating the patient about the disease's signs and symptoms. Dental hygienists must take the time to inform patients with oral cGVHD of their elevated dental caries and oral cancer risks while educating them on risk reduction. Dental hygienists must counsel patients on the importance of regular oral mucosal exams and adherence to the recommended continuing care interval to monitor for signs and symptoms of disease. The dental team should work collaboratively with the patient's medical team to optimize care coordination and maximize oral health outcomes for these unique and complex patients.
Disclosure
This work was supported by the National Institute of Dental and Craniofacial Research grant number R01 DE028336-01A1. Funders did not have any role in data collection, interpretation, and reporting. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Lisa Bennett Johnson, RDH, MS, MPH is a research dental hygienist at Brigham and Women's Hospital and adjunct faculty in the graduate program at the Forsyth School of Dental Hygiene and the Master of Public Health Program at MCPHS University, Boston, MA, USA.
Uhlee Oh, RDH, MSDH is a clinical associate professor at the Forsyth School of Dental Hygiene at MCPHS University and a clinical research dental hygienist at Brigham and Women's Hospital, Boston, MA, USA.
Marilynn Rothen, RDH, MS is a clinical professor of Oral Health Sciences and Research Implementation Manager of the Regional Clinical Dental Research Center, University of Washington School of Dentistry, Seattle, WA, USA.
Herve Y. Sroussi, DMD, PhD is the Director of Research, Division of Oral Medicine and Dentistry, Brigham and Women's Hospital and an associate professor of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA.
David Dean, DDS, MSD is a clinical associate professor and the Graduate Program Director, University of Washington School of Dentistry and the Director of Oral Medicine Services, Seattle Cancer Care Alliance, Seattle, WA, USA.
C. Michele Lloid, RDH, MS is a clinical associate professor of Oral Medicine and Oral Health Sciences, University of Washington School of Dentistry and Clinician/Manager Oral Medicine Service Seattle Cancer Care Alliance, Seattle, WA, USA.
Katelyn Cintron, BS is a research assistant, Brigham and Women's Hospital, Boston, MA, USA.
Stephanie J. Lee, MD, MPH is the Associate Director and a professor in the Division of Clinical Research, Fred Hutchinson Cancer Research Center, Division of Medical Oncology, University of Washington, Seattle, WA, USA.
Corey Cutler, MD, MPH is the Medical Director, Stem Cell Transplantation, Dana-Farber Cancer Institute and an associate professor of Medicine, Harvard Medical School, Boston, MA, USA.
Nathaniel S. Treister, DMD, DMSc is the Chief of the Division of Oral Medicine and Dentistry, Clinical Director of Oral Medicine and Oral Oncology, Dana-Farber/Brigham and Women's Hospital and an associate professor of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, MA, USA.
References
1. Juric MK, Ghimire S, Ogonek J, et al. Milestones of hematopoietic stem cell transplantation - from first human studies to current developments. Front Immunol. 2016 Nov; 7:470.
2. Phelan R, Arora, M., Chen, M. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. Center for International Blood & Bone Marrow Transplant Research, 2020.
3. D'Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017 Sep;23(9):1417-21.
4. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010 Nov;363(22):2091-101.
5. Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011 Jun;29(16):2230-9.
6. Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010 Feb;28(6):1011-6.
7. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015 Mar;21(3):389-401.
8. Georges GE, Bar M, Onstad L, et al. Survivorship after autologous hematopoietic cell transplantation for lymphoma and multiple myeloma: late effects and quality of life. Biol Blood Marrow Transplant. 2020 Feb(2):407-12.
9. Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012 Mar;18(3):348-71.
10. Léger CS, Nevill TJ. Hematopoietic stem cell trans-plantation: a primer for the primary care physician. CMAJ. 2004 May;170(10):1569-77.
11. Jenq RR, van den Brink MRM. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010 Mar;(3):213-21.
12. Martinez-Cibrian N, Zeiser R, Perez-Simon JA. Graft-versus-host disease prophylaxis: pathophysiology-based review on current approaches and future directions. Blood Rev. 2021 Jul;48.
13. Elad S, Raber-Durlacher JE, Brennan MT, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer. 2015 Jan;23(1):223-36.
14. Chaudhry HM, Bruce AJ, Wolf RC, et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant. 2016 Apr;22(4):605-16.
15. Rankin KV, Epstein J, Huber MA, et al. Oral health in cancer therapy. Tex Dent J 2009 May;126(5):389-97, 406-19, 422-37.
16. Arora M, Klein JP, Weisdorf DJ, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. 2011 Jan;117(24):6714-20.
17. Meier JKH, Wolff D, Pavletic S, et al. Oral chronic graft-versus-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Investig. 2011 Apr(2):127-39.
18. Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017 Jan;129(1):30-7.
19. Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013 Oct;19(10):1498-501.
20. Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. the 2014 response criteria working group report. Biol Blood Marrow Transplant. 2015 Jun;21(6):984-99.
21. Bar M, Sandmaier B, Inamoto Y, et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant. 2013 Jun;19(6):949-57.
22. Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002 Aug 15;100(4):1192-200.
23. Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29-52.
24. Tiercy JM. HLA-C incompatibilities in allogeneic unrelated hematopoietic stem cell transplantation. Front Immunol. 2014 May;5:216.
25. Wolff D, Lawitschka A. Chronic graft-versus-host disease. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham (CH): Springer; 2019. Chapter 44, Chronic Graft-Versus-Host Disease; p. 331-45.
26. Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006 Jan;43(1):3-10.
27. Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017Feb;23(2):211-34.
28. Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH consensus criteria. Blood. 2011 Oct;118(15):4242-9.
29. Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134-41.
30. Mays JW, Fassil H, Edwards DA, et al. Oral chronic graft-versus-host disease: current pathogenesis, therapy, and research. Oral Dis. 2013 May;19(4):327-46.
31. Treister N, Duncan C, Cutler C, Lehmann L. How we treat oral chronic graft-versus-host disease. Blood. 2012 Oct;120(17):3407-18.
32. Treister N, Chai X, Kurland B, et al. Measurement of oral chronic GVHD: results from the chronic GVHD consortium. Bone Marrow Transplant. 2013 Aug;48(8):1123-8.
33. Fassil H, Bassim CW, Mays J, et al. Oral chronic graft-vs.-host disease characterization using the NIH scale. J Dent Res. 2012 Jul;91(7 Suppl):45s-51s.
34. Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. the 2014 ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant. 2015 Jul;21(7):1167-87.
35. Hseih R, de Souza, MM, de Paula, F, et al. Oral chronic graft-versus-host disease: a short review. Trends transplant. 2016 Jan; 91(1):1-4.
36. Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. 2008 Jan;52(1):79-109.
37. Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997 Mar;336(13):897-904.
38. Rizzo JD, Curtis RE, Socié G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009 Jan 29;113(5):1175-83.
39. Haverman TM, Raber-Durlacher JE, Raghoebar, II, et al. Oral chronic graft-versus-host disease: what the general dental practitioner needs to know. J Am Dent Assoc. 2020 Nov;151(11):846-56.
40. Dowd FJ. Saliva and dental caries. Dent Clin North Am. 1999 Oct;43(4):579-97.
41. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007 Jan 6;369(9555):51-9.
42. Mawardi H, Hashmi SK, Elad S, et al. Chronic graft-versus-host disease: Current management paradigm and future perspectives. Oral Dis. 2019 May(4):931-48.
43. Mawardi H, Elad S, Correa ME, et al. Oral epithelial dysplasia and squamous cell carcinoma following allogeneic hematopoietic stem cell transplantation: clinical presentation and treatment outcomes. Bone Marrow Transplant. 2011 Jun;46(6):884-91.
44. Bassim CW, Fassil H, Mays JW, et al. Validation of the National Institutes of Health chronic GVHD oral mucosal score using component-specific measures. Bone Marrow Transplant. 2014 Jan(1):116-21.
45. Gomes AO, Torres SR, Maiolino A, et al. Early and late oral features of chronic graft-versus-host disease. Rev Bras Hematol Hemoter. 2014 Jan-Feb;36(1):43-9.
46. Bollero P, Passarelli PC, D'Addona A, et al. Oral management of adult patients undergoing hematopoietic stem cell transplantation. Eur Rev Med Pharmacol Sci. 2018 Feb;22(4):876-87.
47. Ashe TE, Elter JR, Southerland JH, et al. North Carolina dental hygienists' assessment of patients' tobacco and alcohol use. J Dent Hyg. 2005 Spring;79(2):9.
48. Kuten-Shorrer M, Woo SB, Treister NS. Oral graft-versus-host disease. Dent Clin North Am. 2014 Apr;58(2):351-68.
49. Lewis MAO, Williams DW. Diagnosis and management of oral candidosis. Br Dent J. 2017 Nov;223(9):675-81.
50. Quindós G, Gil-Alonso S, Marcos-Arias C, et al. Therapeutic tools for oral candidiasis: current and new antifungal drugs. Med Oral Patol Oral Cir Bucal. 2019 Mar;24(2):e172-80.
51. Singhal S, Powles R, Treleaven J, et al. Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total-body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk Lymphoma. 1997 Feb;24(5-6):539-43.
52. Couriel D, Carpenter PA, Cutler C, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. the 2014 ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant. 2006 Apr;12(4):375-96.
53. Carpenter PA, Schubert MM, Flowers ME. Cevimeline reduced mouth dryness and increased salivary flow in patients with xerostomia complicating chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006 Jul;12(7):792-4.
54. Von der Fehr FR, Löe H, Theilade E. Experimental caries in man. Caries Res. 1970;4(2):131-48.
55. Moynihan P, Petersen PE. Diet, nutrition, and the prevention of dental diseases. Public Health Nutr. 2004 Feb;7(1a):201-26.
56. Castellarin P, Stevenson K, Biasotto M, et al. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol Blood Marrow Transplantation. 2012 Oct;18(10):1573-79.
57. Weyant RJ, Tracy SL, Anselmo TT, et al. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc. 2013 Nov;144(11):1279-91.
58. Featherstone JDB, Chaffee BW. The evidence for caries management by risk assessment (CAMBRA®). Adv Dent Res. 2018 Feb;29(1):9-14
59. Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: a report from the American Dental Association. J Am Dent Assoc. 2018 Oct;149(10):837-49.
60. Contreras V, Toro MJ, Elías-Boneta AR, Encarnación-Burgos A. Effectiveness of silver diamine fluoride in caries prevention and arrest: a systematic literature review. Gen Dent. 2017 May-Jun;65(3):22-9.
61. Bernabé E, Vehkalahti MM, Sheiham A, et al. Sugar-sweetened beverages and dental caries in adults: a 4-year prospective study. J Dent. 2014 Aug;42(8):952-8.
62. Young DA, Lyon L, Azevedo S. The role of dental hygiene in caries management: a new paradigm. J Dent Hyg. 2010 Summer;84(3):121-9.
63. Reed DN, Hall DL, Cottle JH, et al. Dental management of scleroderma patients using pentoxifylline plus vitamin E with and without TheraBite(®) to reduce trismus: two case reports and brief review of literature. Clin Case Rep. 2020 Jan 17;8(2):247-53.
64. Tolle SL. Scleroderma: considerations for dental hygienists. Int J Dent Hyg. 2008 May;6(2):77-83.
65. Poole J, Conte C, Brewer C, et al. Oral hygiene in sclero-derma: the effectiveness of a multi-disciplinary intervention program. Disabil Rehabil. 2010;32(5):379-84.