You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Among the most commonly requested esthetic procedures in dentistry, teeth-whitening treatments have been immensely popular with patients worldwide for many years.1-3 A plethora of both at-home and in-
office procedures are available on the market today, yet the risk of side effects such as tooth sensitivity and gingival irritation remain problematic for many patients. Mitigation of side effects is therefore an important aspect of the management of teeth-whitening treatments to reduce the potential risks and optimize the results of these treatments.3
THE SCIENCE OF TEETH WHITENING
Human teeth have three main layers: enamel, dentin, and pulp. Human enamel is primarily composed of hydroxyapatite, and the basic unit of enamel is the enamel rod, which is made up of tightly packed, organized hydroxyapatite crystals. Underlying the enamel layer is the dentin, composed of 70% hydroxyapatite by weight, 20% organic material, and 10% water.
The enamel layer, which is no more than 2.5 mm in thickness, is thickest at the cusp or incisal edge and thinnest at the cemento-enamel junction.3 The enamel layer is acellular but contains proteins and is honeycombed with voids in what is known as the enamel matrix. These voids do not stay empty, and over time become filled with long-chained organic compounds, or chromogens, from the foods and liquids that are ingested. It is these chromogens that stain the teeth as they accumulate over time on the tooth.3 Also, as a person ages, the enamel layer wears down and thins out, causing the yellower dentin layer beneath the enamel to become more visible.3
Peroxide Activation: Increasing the Production of Free Radicals
Hydrogen and carbamide peroxides release oxygen free radicals that oxidize long-chain organic compounds3 (ie, the chromogens) found within the pores of the enamel's matrix. When the enamel is treated with these oxidizing agents during a teeth-whitening or bleaching procedure, the chromogen long-chain molecules responsible for the discoloration are broken by high-energy free radicals that are released by the chemical oxidation process.3
In an electron-spin-resonance study, more hydroxyl radical
(*OH) was detected as the H2O2 concentration was increased. It is suggested that H2O2 and *OH do not influence the inorganic tissue of dentin but attack the organic component of dentin. These facts suggest that *OH has the primary role in tooth bleaching with H2O2.4
Heat activation of peroxide increases the formation of the hydroxyl (*OH) and perhydroxyl (HOO*) free radicals. Each temperature increase of 10ºC increases the chemical reactivity by 230%.5 As temperature is increased, the diffusion of hydrogen peroxide through enamel and dentin is also increased.6
FACTORS ASSOCIATED WITH THE EFFECTIVENESS AND SAFETY OF TEETH-WHITENING TREATMENTS
Whitening effectiveness and safety is based on the following:
• Gel concentration and type of whitening agent
(hydrogen versus carbamide peroxide)7
• Exposure (dwell) time of the whitening agent on teeth8
• Intrapulpal temperature of the teeth from the heated
whitening agent9
• Activation of the whitening agent (free radical production)9,10
• Penetration of the whitening agent into the enamel matrix6,9
Whitening efficacy is concentration and time dependent. As shown in Table 1, there is a significant difference between carbamide peroxide-based and hydrogen peroxide-based whitening gels, with carbamide peroxide being only 36% as potent as hydrogen peroxide for a given concentration.11 Additionally, the concentrations of carbamide peroxide and hydrogen peroxide differ vastly depending on which of the two treatment protocols (at-home whitening trays or in-office whitening treatments) is
used (Table 1). The typical exposure times are 7 to 30 or more hours with at-home whitening treatments and 30 to 60 minutes with in-office treatments.11,12
ADVERSE EFFECTS
Common side effects for all whitening treatments are gingival irritation and tooth sensitivity; the degree to which these side effects occur varies depending on the exposure time, whether carbamide or hydrogen peroxide is used, and the concentration of peroxide, with higher concentrations associated with greater adverse effects.13-17
Tooth Sensitivity and Gingival Irritation
Tooth sensitivity during bleaching has been the most common adverse effect, occurring in as many as 78% of patients, according to some studies.16,18 This side effect usually begins during treatment, and it may last for several days.3 One cause of tooth sensitivity is dehydration or desiccation of the teeth, which can result from some in-office whitening treatments.19 Gingival irritation results from contact with the peroxide, and has been noted to occur when bleaching trays have been poorly fabricated.20 This soft tissue irritation tends to begin within 1 day of treatment and, like tooth sensitivity, can persist for several days.3
Using lower concentrations of carbamide or hydrogen peroxide is associated with reduced tooth sensitivity and soft tissue irritation. Dental professionals can also reduce the potential for these side effects through additional measures, such as gingival isolation techniques. With in-office treatments, the use of in-office gingival isolation techniques prevents the soft tissues from coming into contact with the peroxide-based tooth gels, and shorter treatment times may also decrease the likelihood of sensitivity.
Dehydration
The widely accepted hydrodynamic theory propounded by Brannstrom21,22 discusses how pain can be elicited in the pulpal nerve tissue from different stimuli, especially dehydration of the dentinal tubule's intracellular fluid. Markowitz23 describes the dentinal tubules as providing the critical link between most forms of dental stimulation and activation of the intradental nerves. Both of these explanations of dental pain suggest that the dentinal tubules of the teeth should not be dehydrated.
Utilizing an open mouth isolation technique during teeth whitening (Figure 1) can lead to dehydration of the teeth and significant post-treatment tooth sensitivity. The dehydration effect has been found to occur when a rubber dam is used to isolate the teeth for an hour or more,19 which is the approximate duration of standard in-office whitening treatments. The use of intense light exposure (heat generated by the light) has also been noted to increase dehydration.19 In addition, in a study comparing a chemically activated teeth-whitening treatment with a light-activated treatment, it was reported that light activation did not increase the whitening result.24
Studies have shown that dehydrated-state tooth color values can significantly differ immediately after in-office teeth-whitening treatment, with the desiccated teeth appearing significantly whiter, only to darken (relapse) quickly (over a period of 1 to 2 weeks) once they have regained hydration when bathed with saliva in the mouth.16,25 Following the in-office treatment, the patient may be provided with take-home whitening trays to counter-
act the shade relapse that occurs.26
TEETH-WHITENING METHODS: OVER-THE-COUNTER, TAKE-HOME, AND IN-OFFICE
The three categories of teeth-whitening methods recognized by the American Dental Association are over-the-counter, take-home, and in-office treatments.27 The choice of treatment not only directly influences the speed and effectiveness of the
teeth-whitening process, it can also impact result sustainability, while some whitening treatments may be associated with a greater degree of side effects than others, frequently as a result of the peroxide concentration of the whitening gel.28
Over-the-Counter Treatments and Take-Home Trays
Over-the-counter (OTC) whitening treatments have enjoyed increased popularity in recent years owing to their wide availability and consumers' easy access to these products,29 which are available in most drug stores and supermarkets. Because of the lower concentrations of peroxide used in these products, longer treatment times are required.3
Unlike OTC products, take-home whitening trays are provided by a dental professional, who examines the patient and fabricates the custom-fitted trays.18 The whitening gel in take-home trays has a lower concentration of carbamide or hydrogen peroxide than in in-office treatments, but they need to be used over a longer period of time, with the prolonged exposure increasing the likelihood of side effects such as tooth sensitivity.20
In-Office Treatments
In-office treatments yield more rapid results than at-home whitening treatments.20 Examples of in-office treatments include Philips® Zoom (Philips), Opalescence™ Boost™ (Ultradent), and Thera-
Smile® Whitening system (Mavrik Dental Systems®). As shown in Table 1, in-office whitening procedures use gels with a higher concentration of peroxide, and therefore are ideal for patients who are seeking fast results along with supervision by a dental professional. Because of these higher peroxide concentrations, however, practitioners must take care to mitigate side effect risks, particularly tooth sensitivity and soft tissue irritation.
With most in-office whitening treatments, the whitening gel is manually applied by painting a thin layer of whitening agent (that remains statically applied before removal) onto the buccal surfaces only of the anterior teeth (the lingual surfaces of the teeth remain untreated), usually involving three gel applications and removals per treatment. The whitening agent remains static on the teeth after application and before removal. For this reason, these in-office whitening treatments utilize an open mouth isolation technique to prevent the soft tissues from coming in contact with the peroxide-based treatment gel. Open mouth isolation involves retracting the lips and cheeks, protecting the face and eyes, utilizing saliva ejector/cotton rolls, and placing various light curable resins on the buccal aspects of the gingival ridges. Examples of these light curable resins are Philips® Zoom's Liquidam® (Philips),30Pulpdent Kool-Dam™,31 and OpalDam (Ultradent).32
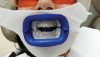
A recently developed in-office whitening treatment provides a fully automated whitening treatment that applies and removes gel with closed mouth isolation (Figure 2).33 The system automatically delivers thermo-chemically activated whitening gel from a pre-filled cartridge, causing the gel to flow onto all surfaces of the upper and lower teeth, which are contained within a vacuum-sealed, soft mouthpiece.33 The vacuum-sealed mouthpiece creates an intraorally isolated environment through which only concentrated treatment fluids can flow, with refreshed, heated fluids continuously delivered in cycles to the targeted treatment area for the prevention of tooth desiccation. Preformed elastomeric silicone gingival ridge barriers cover the upper and lower alveolar gingival ridges (both the buccal and lingual aspects of the ridges) for protection against the whitening gel but leave the teeth exposed; together with the light cured dental resin,34 these provide a fully closed mouth isolation technique to help prevent dehydration of the teeth.35
Conclusion
Although teeth-whitening treatments have been widely available for decades, tooth sensitivity and gingival irritation, the most common side effects of these treatments, remain a problem for many patients. Using reduced concentrations of carba-
mide and hydrogen peroxide is one means of mitigating these adverse effects, but higher concentrations-especially those used with in-office treatments-are associated with improved and more rapid whitening results, the treatment goal of patients and practitioners alike. Most in-office teeth-whitening treatments have utilized an open mouth isolation method to protect the intraoral soft tissues from contact with whitening agents. While this technique reduces the likelihood of gingival irritation, it may also cause tooth dehydration and desiccation, which contribute to the adverse effect of tooth sensitivity. A system that uses a closed mouth isolation technique to prevent dehydration of the teeth is one method practitioners may consider to help reduce the risks of both gingival irritation and tooth sensitivity in their patients who opt for in-office treatment. Finally, careful supervision of the whitening procedure is key to patient safety and optimal results.
References
1. Calderini A, Sciara S, Semeria C, Pantaleo G, Polizzi E. Comparative clinical and psychosocial benefits of tooth bleaching: different light activation of a 38% peroxide gel in a preliminary case-control study. Clin Case Rep. 2016;4(8):728-735.
2.Bizhang M, Domin J, Zimmer D. Effectiveness of a new non-
hydrogen peroxide bleaching agent after single use - a double-
blind placebo-controlled short-term study. J Appl Oral Sci. 2017;25(5):575-584.
3. Carey CM. Tooth whitening: what we now know. J Evid Based Pract. 2014;(14 Suppl):70-76.
4. Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod. 2004;30(1):45-50.
5.Johns D, Hutton A. The Arrhenius Law - Arrhenius Plots. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/06%3A_Modeling_Reaction_Kinetics/6.02%3A_Temperature_Dependence_of_Reaction_Rates/6.2.03%3A_The_Arrhenius_Law/6.2.3.04%3A_The_Arrhenius_Law_-_Arrhenius_Plots. Updated September 20, 2020. Accessed May 26, 2022.
6. Peterson BK. Quantitative analysis of the diffusion of hydrogen peroxide through teeth [master's thesis]. Los Angeles: University of California; 2012. Available at: https://escholarship.org/uc/item/2kx225fr. Accessed March 15, 2022.
7.Féliz-Matos L, Hernandez LM, Abreu N. Dental bleaching techniques; hydrogen-carbamide peroxides and light sources for activation, an update. Open Dent J. 2014;8:264-268.
8. Bowles WH, Ugwuneri Z. Pulp chamber penetration by
hydrogen peroxide following vital bleaching procedures. J Endod. 1987;13(8):375-377.
9.Batista GR, Barcellos DC, Borges AB, Torres CRG. Analysis of the pulp chamber temperature of teeth submitted to light activation with and without bleaching gel. World J Dent. 2011;2(1):23-27.
10.Davidi MP, Hadad A, Weiss EI, Domb A, Mizrahi B, Sterer N.
The effect of a mild increase in temperature on teeth bleaching. Quintessence Int. 2008;39(9):771-775.
11.Bassett JL. Tooth whitening. Inside Dentistry. 2017;13(11):63-68.
12.Bernadon JK, Ferrari P, Baratieri LN, Rauber GB. Comparison of treatment time versus patient satisfaction in at-home and in-office tooth bleaching therapy. J Prosthet Dent. 2015;114(6):826-830.
13.Bruzell EM, Pallerson U. Side effects of external tooth bleaching: a multi-centre practice-based prospective study. Br Dent J. 2013;215(9):E17.
14.Auschill TM, Hellwig E, Schmidale S, Sculean A, Arweiler NB . Efficacy, side-effects and patients' acceptance of different bleaching techniques (OTC, in-office, at-home). Oper Dent. 2005;30(2):
156-163.
15. Goldberg M, Grootveld M, Lynch E . Undesirable and adverse effects of tooth-whitening products: a review. Clin Oral Investig.
2010;14(1):1-10.
16.Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200(7):371-376.
17. Leonard RH Jr, Garland GE, Eagle JC, Caplan DJ. Safety issues when using a 16% carbamide peroxide whitening solution. J Esthet Restor Dent. 2002;14(6):358-367.
18.Strassler HE. At-home vital tooth bleaching. Inside Dentistry. 2011;7(2). www.dentalaegis.com/id/2011/02/at-home-vital-toothbleaching-a-current-status-update-on-professionally-dispensedand-otc-methods. Accessed April 21, 2022.
19. Haywood VB. In-office bleaching: lights applications and
outcomes. Current Pract. 2009;16(4):3-6.
20.Levine LB. Teeth-whitening technology. Inside Dentistry. 2017;13(3). https://www.aegisdentalnetwork.com/id/2017/03/teeth-whitening-technology. Accessed April 21, 2022.
21.Brannstrom M. The hydrodynamic theory of dentinal pain:
sensation in preparations, caries, and the dentinal crack syndrome.
J Endod. 1986;12(10):453-457.
22. Milagny S, Aggarwal V, Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13(4):218-224.
23. Markowitz K. Pretty painful: why does tooth bleaching hurt? Med Hypotheses. 2010;74(5):835-840.
24.Kugel G, Papathasiou A, Williams AJ, Anderson C, Ferreira S. Clinical evaluation of chemical and light-activated tooth whitening systems. Compend Contin Educ Dent. 2006;27(1):54-62.
25. Matis BA, Cochran MA, Franco M, Al-Ammar W, Eckert GJ, Stropes M. Eight in-office tooth whitening systems evaluated in vivo: a pilot study. Oper Dent. 2007;32(4):322-327.
26. Deliperi S, Bardwell DN, Papathanasiou A. Clinical evaluation of a combined in-office and take-home bleaching system. J Am Dent Assoc. 2004;135(5):628-634.
27.Department of Scientific Information, Evidence Synthesis & Translation Research, ADA Science & Research Institute, LLC.
Whitening. ADA website. www.ada.org/en/member-center/oral-health-topics/whitening. Updated October 30, 2020. Accessed April 21, 2022.
28. Leonard RH Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997; 28(8):527-534.
29. Omar F, Ab-Ghani Z, Rahman NA, Halim MS. Nonprescription bleaching versus home bleaching with professional prescriptions: which one is safer? A comprehensive review of color changes and their side effects on human enamel. Eur J Dent.2019;13(4):589-598.
30. Philips Zoom WhiteSpeed Light-Accelerated Tooth Whitening Procedure Kit [Instructions for Use]. Ontario, CA: Discus Dental, LLC. https://www.documents.philips.com/assets/20201026/a00ebaf5659242a6ba90ac6000727202.pdf. Accessed April 5, 2022.
31. Pulpdent Kool-Dam. http://pulpdent.com/pulpent-products/kool-dam. Accessed May 15, 2022
32.Ultradent Opaldam. https://www.ultradent.com/products/categories/whitening/isolation/opaldam-and-opaldam-green. Accessed May 31, 2022.
33. TheraSmile® Powered by iowave™ technology. Mavrik website.http://mavrikdental.com/science. Accessed March 30, 2022.
34. Gingival isolation. Mavrik website. http://mavrikdental.com/products/therasmile-system. Accessed April 25, 2022.
35. TheraSmile®Vacuum-sealed hydrated environment. http://mavrikdental.com/products/therasmile-system. Accessed
April 5, 2022.