You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Lyme disease, the most common vector-borne disease in the United States (US), is transmitted by ticks infected with Borrelia burgdorferisensu stricto (Bbss) or Borrelia mayonii.1 Carriers of the pathogen include Ixodes scapularis(commonly known as the deer tick or blacklegged tick), and Ixodes pacificus(commonly known as the western blacklegged tick).2 The Bbss spirochetal bacterium is transferred to human hosts during the blood meal of an infected tick, with the incubation period in humans, prior to symptom onset, ranging from three to thirty days.3,4 First recognized in Connecticut in the 1970s, Lyme disease was added to the National Notifiable Disease Surveillance System in 1991.1,5 Since then, the number of reported cases and geographical distribution of the disease has quickly spread across the country and is now considered endemic in 14 states including Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, and Wisconsin.1,5 States that share a border or are located between high endemic states, are classified as "neighboring states" and are also showing an increased incidence of confirmed cases.5 Over 275,000 cases of Lyme disease were reported to the Centers for Disease Control (CDC) from 2008-2015.5 Currently, state health departments report 30,000 new cases of Lyme disease to the CDC each year2; however, the number of reported cases is estimated to be much lower than the number of diagnosed cases - potentially by ten-fold.1,6 The increasing incidence of this infectious disease is of growing concern, and as members of interdisciplinary health care teams oral health care professionals need to be aware of the signs and symptoms.
Lyme disease affects multiple body organs, and if left untreated can potentially be life threatening; obtaining an early diagnosis through interprofessional care is critical. The CDC is asking all health care providers to educate themselves about the full range of the presenting signs and symptoms of Lyme disease to minimize the morbidity of the disease.3,4 When diagnosed and treated early, patients can be successfully cured and report fewer residual complications.5 However, obtaining an early diagnosis for this complex disease is difficult in many cases due to the limitations of serological tests coupled with the onset of signs and symptoms that can vary significantly from person-to-person and also mimic a multitude of other ailments depending on the dissemination stage.3,4,6
The localized early stage may appear within 3-30 days after an infected tick bite and typically begins with an expanding skin lesion, erythema migrans, commonly known as the bull's eye rash.4 Erythema migrans is considered to be a critical sign of Lyme disease and is often used to make a clinical diagnosis. However, the rash only appears in 70-80% of cases and may be missed if it presents on the back half of the body, on darker skin tones, or has an atypical appearance.4 Early stages may also include fever, chills, headache, fatigue, muscle and joint aches, and lymphedema.4 During the disseminated stage, seven days to several months after the initial infection, signs and symptoms may include severe headaches, neck stiffness, additional erythema migrans lesions, arthritis, and facial palsy.4 Individuals may also experience nerve pain, problems with short-term memory, irregular heartbeat, inflammation of the brain and spinal cord, shooting pains, numbness, and/or tingling in the hands or feet.4 The signs and symptoms may appear atypical or not at all, further complicating the ability to obtain a timely diagnosis. Orofacial manifestations of Lyme disease have appeared in the health care literature, but they have not been a research focus despite reports indicating that orofacial manifestations may be the first signs and symptoms to appear.7 The possibility of Lyme disease should be a consideration for oral health care professionals when patients present with unexplained facial paralysis,8-17 neck stiffness,11,13,15,18 headache,8-13,15-19 TMJ pain,10,13 altered taste,19 sore throat,12 and neck pain,10,12,13,17,19when a clinical examination fails to identify a specific oral health pathology.
The orofacial manifestations of Lyme disease affect head and neck anatomical areas that are routinely examined by oral health care professionals. A 2014 study found that 46% of surveyed health care providers had encountered a Lyme disease patient, and the researchers concluded that health care providers in general required more education about Lyme disease in order to promote an early diagnosis.20 This also points to the possibility that many dental professionals may be unaware of the orofacial manifestations linked to Lyme disease. This missed opportunity for making a timely diagnosis may allow the disease to progress to later stages characterized by chronic suffering and life-threatening conditions that are difficult to treat, negatively affecting the patient's quality of life. Furthermore, patients often experience frustration when seeking a diagnosis due to health care professionals who may be disrespectful and dismissive because of not being well educated in the signs and symptoms of Lyme disease.21 It is important for dental professionals to be aware of the orofacial manifestations of Lyme disease and to have a referral and follow-up plan with primary care physicians to achieve the best possible health outcomes for their patients. The purpose of this systematic review was to examine the literature to identify the frequencies of orofacial manifestations documented in Lyme disease patients in the US to help inform oral health care providers of the orofacial manifestations of the disease.
Methods
This systematic review followed the guidelines set forth in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).22 An electronic search of the literature was conducted by a university health and life sciences librarian from May 2019 until October 2019 and included the following databases: Dentistry and Oral Sciences Source (EBSCO), PubMed (first search), Cinahl Plus with Full Text (EBSCO), and Medline (first search) for articles published from January 1990 to October 2019. Several search term alterations were used and synonyms for the key search terms were cross-checked using the United States National Library of Medicine Unified Medical Language System Metathesaurus®.23 The search strategy used keywords and MeSH terms that included terminology and synonyms for Lyme disease, vector, pathogen, head/neck anatomical landmarks, and orofacial manifestations. The search was limited to peer-reviewed journals, articles which included populations of Lyme disease patients diagnosed in accordance with CDC protocol in the US, and full-text articles in the English language. Articles were included if they met the following criteria: 1) Studies that include populations from the US, 2) available in full-text and in the English language, and 3) confirmed CDC diagnosis of Lyme disease. Exclusion criteria included: 1) studies of non-US populations, 2) studies involving animal subjects, 3) tick-borne diseases other than Lyme disease, and 4) studies that did not confirm a Lyme disease diagnosis.
A citation managing system (EndNote X9; Clarivate Analytics, Philadelphia, PA) was utilized to organize identified articles. There were no limits on study designs, but grey literature and letters to editors were excluded. Retrieved articles were independently reviewed for appropriateness based on titles and abstracts by two researchers (BB and KJ). Meetings for discussion were held to resolve disagreements that were settled by consensus. Risk of bias was assessed independently by BB and KJ, and data extraction was completed using a modified version of the Cochrane Data Collection Form for Randomized Control Trials and Non-randomized Control Trials and meetings were held to resolve disagreements by consensus.
Results
An initial search of the databases produced a total of 217,381 results after filters and limits based on inclusion/exclusion criteria were applied. The titles and abstracts were further filtered based on exclusionary criteria, resulting in 744 articles to be reviewed. Ninety-five were removed due to duplication, yielding 649 articles. Two reviewers (BB and KJ) independently screened each of the 649 articles by titles and abstracts to identify additional articles that should be excluded due to a lack of relevancy; disagreements were settled by consensus, resulting in the removal of 441 articles. Exclusions based on titles and abstracts were made for the following reasons: published before 1990, orofacial manifestations were not addressed, articles were not accessible, letters to editors, not peer-reviewed, full text not available, only available as a research poster, and non-human research. The remaining full-text articles (n=208) were further independently reviewed based on inclusion/exclusion criteria and risk of bias, resulting in the removal of 196 articles after disagreements were settled by consensus. Articles were rated as poor, fair, or good based on the risk of bias tool (Table I). Articles removed due to risk of bias (n=31) included case studies, and literature reviews that did not report data collected from human subjects. Items determined to be "fair" or "poor" were included if relevant data was reported. Articles removed for not meeting the inclusion criteria (n=165) included those that were foreign, did not follow CDC serological protocol, and articles that did not report results of Lyme disease from patients with orofacial manifestations. Clinical trials meeting inclusion/exclusion criteria were not identifiable.
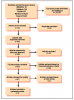
A total of twelve articles were judged as being acceptable for inclusion in the systematic review for synthesis. Seven (58%) of the included studies were from the 1990s,10-13,16,17,19 three (25%) from the 2000s,8,15,18 and two (17%) were from the 2010s.9,14 Publication sources for the twelve articles included several journals: The Journal of Clinical Microbiology(2),18,19 The New England Journal of Medicine(2),11,12 Pediatrics(2),8,9 The American Journal of Otology(1),16 Otology & Neurotology(1),15 The Journal of Pediatric Infectious Diseases Society(1),14 The American Journal of Medicine(1),13 Neurology(1),17 and The American Journal of Otolaryngology(1).10 The PRISMA flow chart of the results of included and excluded studies is shown in Figure 1.
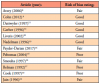
Data extraction of the included articles was completed independently by BB and KJ to include the first author, year, demographic information of the study populations, confirmation of Lyme disease, and presence of orofacial manifestations. Articles included for synthesis documented orofacial manifestations of Lyme disease in human patient populations in the endemic states of Delaware (25%, n=3),8-10 Massachusetts (33%, n=4),9,11,14,15 Pennsylvania (17%, n=2),9,10 New Jersey (8%, n=1),10 Connecticut (25%, n=3),12,15,19 and New York (42%, n=5).13,16-19 Demographic characteristics of the patient populations varied in the twelve studies. All articles included both males and females, however nine included mostly males (75%, n=9),8-13,15-17 only two articles were predominately of female populations (17%, n=2),18,19 and one article did not make a clear distinction.14 The age ranges of the studied populations also varied. Six articles included a mixture of ages from childhood to adulthood (50%, n=6),10-13,15,16,19 four included children and adolescents (33%, n=4),8,9,14,17 and one focused only on adults (8%, n=1).18 Six of the studies were retrospective (50%, n=6),8-10,14-16 five were prospective (42%, n=5),11-13,18,19 and one followed an observational design (8%, n=1).17 In half of the articles (50%, n=6), the number of times orofacial manifestations appeared was more than that of the number of total patients10,11,15-17,19; in one article they appeared the same number of times (8%, n=1),14 and in five articles they appeared only slightly less (42%, n=5).8,9,12,13,18 Patient demographics and the frequency of orofacial manifestations by article are shown in Table II.
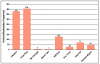
Eight orofacial manifestations were reported among the included articles: headache,8-13,15-19 facial palsy,8-17,19 temporomandibular joint arthralgia,10,13 altered taste,19 stiff neck,11,13,15,18 sore throat,12 neck pain/arthralgia,10,12,13,17 and erythema migrans rash on the head or neck.12 The frequencies of orofacial manifestations per study are shown in Figure 2. Among the twelve included articles, there were a total of 951 confirmed cases of Lyme disease. The frequencies of the eight orofacial manifestations among those confirmed cases were reported as follows: headache (39.5%, n=376),8-13,15-19 facial palsy (42.5%, n=404),8-17,19 temporomandibular joint arthralgia (.42%, n=4),10,13 altered taste (.11%, n=1),19 stiff neck (13.6%, n=129),11,13,15,18 sore throat (3.0%, n=29),12 neck pain (7.5%, n=71),12,13,17,19 and erythema migrans rash on the head or neck (5.2%, n=49).12 The frequencies of orofacial manifestations per study are shown in Table III a, Table b.
Discussion
The scientific literature was systematically reviewed to synthesize information regarding the orofacial manifestations of Lyme disease that would be most relevant to oral health care providers. Studies that met the inclusion criteria from 1992-2017 were published in journals that do not target the dental professional audience. There is a need for this timely research to appear in dental and dental hygiene journals so that the oral health care community is informed regarding the various ways this growing infectious disease may be manifested within their patient populations. Additionally, none of the included research studies discussed clinical assessments or scales that could be used to quantify the severity of Lyme disease related orofacial manifestations. Such diagnostic tools along with recommendations on how to best manage the orofacial manifestations to ease discomfort, improve function, and track recovery or relapse would be beneficial to oral health care professionals.
Fewer than one half (n=6) of the US endemic states (n=14) were represented in the included studies. More studies are needed in the literature from other endemic and neighboring states, especially since the CDC Morbidity and Mortality Weekly Report concluded that incidence rates are increasing in neighboring states.5 Oral health care providers who practice in those geographic areas need to have evidence-based information regarding how to care for affected patient populations. The literature also does not report orofacial manifestations evenly across demographics. Since disease transmission does not discriminate between genders, studies are needed to fully represent both males and females. An underrepresentation of females in the literature may be of concern especially for those residing in low incidence states, since Lyme disease has been found to be more common among females in states with low incidence rates.5
A total of eight orofacial manifestations were documented in the included studies. Half of the studies reported higher frequencies of these manifestations than the total number of study participants likely due to participants who experienced multiple oral manifestations. Regarding patients who present with orofacial symptoms of unknown origin, oral health care providers should carefully document their findings following a thorough health history interview and extra/intraoral examination. Questions that may assist dental hygienists and dentists while conducting a health history interview when Lyme disease infection is suspected could include: "Have you noticed a red, circular skin rash?", "Have you worked or played outdoors within the last month?", "Do you recall a recent insect bite?", "Have you recently traveled to states known to be endemic for Lyme disease?", and "Have you been tested by your medical physician for Lyme disease?"4 Occurrences of these orofacial manifestations should be taken into consideration for any type of differential diagnoses when the typical oral pathologies of trauma or infections cannot be identified. This is particularly important since these presenting signs and symptoms can occur during the localized early stage of Lyme disease when serology testing may fail.
Eight of the included studies reported headache, neck pain/arthralgia, sore throat, facial paralysis, stiff neck, altered taste, and TMJ arthralgia as orofacial manifestations appearing during the early stage of disease,11-15,17-19 and two studies reported headache, neck pain, and sore throat during the late stage of disease.12,17 Four included studies did not specify early or late dissemination for disease stage of the sample populations.8-10,16 While the majority of reported orofacial manifestations appeared during early dissemination among these studies, it is difficult to say this is enough evidence to generalize that all orofacial manifestations primarily occur during early dissemination of the disease. Future research should include specific details about the timeframe of when signs and symptoms appear in the disease process so that a pattern of correlation may be identified over time. Oral health care providers with a keen awareness of the role of these oral manifestations could refer a patient for medical examination when Lyme disease is suspected, increasing the chances of making a timely diagnosis.
A total of 951 patients were confirmed to have Lyme disease across the twelve studies. Headache and facial palsy were reported most frequently while temporomandibular joint arthralgia and altered taste were reported the least. Low frequencies of neck stiffness and temporomandibular joint arthralgia was a surprising finding since the review process revealed several articles and case studies which reported those two symptoms as primary orofacial manifestations.7,24-34 However, case studies were excluded from this systematic review, due to the high for risk of bias. Considering that neck stiffness and temporomandibular joint arthralgia appeared in several case studies and have been recognized by the CDC as Lyme disease symptoms,2 they may merit further study.
Avery et al devised a statistical model to help predict Lyme meningitis and stated that odds ratios for headaches can serve as an independent predictor of the condition and found that headache duration was an independent predictor of Lyme meningitis in children in an endemic region.8 This model allowed practitioners to calculate the probability from .01 to .99 of children having Lyme meningitis.8 Each additional day of headache duration increased the odds ratio of having Lyme disease by 0.136.8 The Avery prediction model was compared against a "rule of 7's" prediction model devised by Cohn et al. This prediction model classifies suspicious cases as "low risk" when 3 of the following criteria are met: "<7 days of headache, <70% cerebrospinal fluid mononuclear cells, and absence of seventh or other cranial nerve palsy."9 Cohn et al reported that both the Avery model and the rule of 7's performed well when applied to a sample population of children. However, the rule of 7's model had higher sensitivity (96% [95% CI: 90%-99%]) compared to the Avery model (83% [95% CI: 75%-89%]), and fewer misclassifications when assessing patient risk for Lyme disease.9
Smouha et al applied a researcher-designed clinical probability scale compared against serology test results to determine if clinical signs and symptoms could accurately assess Lyme disease probability.16 The researchers defined clinical probabilities of Lyme disease signs and symptoms including orofacial manifestations: "definite" probability (erythema migrans rash), "high" probability (headache and stiff neck), "intermediate" probability (arthralgias), and "possible" (headache alone).16 However, they concluded that the majority of disease predictors could not be made solely based on clinical data, which contrasts findings from Avery's prediction model and Cohen's rule of 7's model.16 Risks or odds ratios for orofacial manifestations should be investigated by validated prediction models to determine whether they can assist health care providers who encounter suspicious cases. Since patients presenting with orofacial manifestations are considered medical emergencies, the ability for oral health care providers to apply risk prediction models until serology test results are available may help reduce unnecessary antibiotic prescriptions and invasive medical procedures and support timely referrals.
Generalizability of this systematic review is limited because studies of populations outside of the US were not included as Lyme disease variants in other countries may differ from those found in the US. Additionally, the Cochrane Data Collection Form for Randomized Control Trials and Non-randomized Control Trials was modified as there were few randomized control trials identified in the literature that met the inclusion criteria.
Future research focused on the eight orofacial manifestations associated with Lyme disease as presented in oral health care settings is needed. Additionally, there were other less common orofacial manifestations of Lyme disease reported as case studies that were not included in this review. A systematic review of case studies could be helpful to investigate the phenomenon of these less common manifestations. Case-control studies of integrated electronic medical and dental records could be conducted to investigate the frequencies of orofacial manifestations of confirmed Lyme disease cases against healthy cases from endemic states to support evidence-based decision making for all health care providers. Future studies should also investigate the efficacy of integrated electronic medical and dental records to improve the interprofessional collaboration of health care professionals for the management of patients with Lyme disease.
Conclusion
This systematic review researched orofacial manifestations of Lyme disease that could be recognized by oral health care providers. Eight orofacial manifestations of Lyme disease have been well documented in the literature. Ongoing research regarding the orofacial manifestations of Lyme disease is needed so that this medical phenomenon can be well understood by dental professionals in order to best serve their patients.
Brenda T. Bradshaw, RDH, MSDH is an assistant professor and undergraduate program director, Gene W. Hirschfeld School of Dental Hygiene; Kelsey M. Jones, BS was a student in the Department of Biological Sciences; Joleen M. Westerdale-McInnis, MLIS, MFA is a librarian for the Health and Life Sciences; Holly D. Gaff, PhD is a professor, Department of Biological Sciences and a honorary professor of Mathematics, Statistics and Computer Science at the University of KwaZulu-Natal, Durban, South Africa; all are from Old Dominion University, Norfolk, VA, USA.
References
1. Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 2018 Apr; 34(4): 295-309.
2. Centers for Disease Control and Prevention. Lyme disease: frequently asked questions [Internet]. Atlanta (GA); Centers for Disease Control and Prevention: 2019 [updated 2019 Sep 5; cited 2019 Oct 25]. Available from: https://www.cdc.gov/lyme/faq/
3. Centers for Disease Control and Prevention. Lyme disease [Internet]. Atlanta (GA); Centers for Disease Control and Prevention: 2019 [updated 2019 Feb 6; cited 2019 Oct 25]. Available from: http://www.cdc.gov/lyme/index.html
4. Centers for Disease Control and Prevention. Tickborne diseases of the United States: a reference manual for healthcare providers, 5th ed. [Internet]. Atlanta (GA); Centers for Disease Control and Prevention: 2019 [updated 2019 Jan 10; cited 2019 Oct 10]. Available from: https://www.cdc.gov/ticks/tickbornediseases/index.html
5. Centers for Disease Control and Prevention. Morbidity and mortality weekly report (MMWR) surveillance summaries: surveillance for Lyme disease - United States, 2008-2015; 66(22) [Internet]. Atlanta (GA); Centers for Disease Control and Prevention: 2017 [updated 2017 Nov 10; cited 2018 Nov 1]. Available from https://www.cdc.gov/mmwr/volumes/66/ss/pdfs/ss6622-H.pdf
6. Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: A rigorous review of diagnostic criteria and treatment. J Autoimmun. 2014 Feb; 2015(57): 82-115.
7. Lee DH, Chu PK, King B, Rosenberg D. Limited opening secondary to Lyme disease in an 8-year-old child. J Dent Child. 2009 Jul; 76(2): 165-9.
8. Avery RA, Frank, G, Glutting JJ, Eppes SC. Prediction of Lyme meningitis in children from a Lyme disease-endemic region: A logistic-regression model using history, physical, and laboratory findings. Pediatr. 2006 Jan; 117(1): e1-7.
9. Cohn KA, Thompson AD, Shah SS, et al. Validation of a clinical prediction rule to distinguish Lyme meningitis from aseptic meningitis. Pediatr. 2012 Jan; 129(1): e46-53.
10. Cook SP, Macartney KK, Rose CD, et al. Lyme disease and seventh nerve paralysis in children. Am J Otolaryngol. 1997 Sep-Oct; 18(5): 320-3.
11. Dattwyler RJ, Luft BJ, Kunkel MJ, et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med. 1997 Jul; 337(5): 289-95.
12. Gerber MA, Shapiro ED, Burke GS, et al. Lyme disease in children in southeastern Connecticut. N Engl J Med. 1996 Oct; 335(17): 1270-4.
13. Nadelman RB, Nowakowski J, Forseter G, et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996 May; 100(5): 502-8.
14. Paydar-Darian N, Kimia AA, Lantos PM, et al. Diagnostic lumbar puncture among children with facial palsy in a Lyme disease endemic area. JPIDS. 2017 Jun; 2017(6): 205-8.
15. Peltomaa M, McHugh G, Steere AC. The VlsE (IR6) Peptide ELISA in the serodiagnosis of Lyme facial paralysis. Otol Neurotol. 2004 Sep; 25(5): 838-41.
16. Smouha EE, Coyle PK, Shukri S. Facial nerve palsy in Lyme disease: Evaluation of clinical diagnostic criteria. Am J Otol. 1997 Feb; 18(2): 257-61.
17. Belman AL, Coyle PK, Roque C, Cantos E. MRI findings in children infected by Borrelia burgdorferi. Pediatr Neurol. 1992 Jun; 8(6): 428-31.
18. Liveris D, Wang G, Girao G, et al. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: Correlation of results with clinical and laboratory findings. J Clin Microbiol. 2002 Apr; 40(4): 1249-53.
19. Jain VK, Hilton E, Maytal J, et al. Immunoglobulin M immunoblot for diagnosis of Borrelia burgdorferi infection in patients with acute facial palsy. J Clin Microbiol. 1996 Aug; 34(8): 2033-5.
20. Brett ME, Hinckley AF, Zielinski-Gutierrez EC, Mead PS. US healthcare providers' experience with Lyme and other tick-borne diseases. Ticks Tick Borne Dis. 2014 Jun; 5(4): 404-8.
21. US Department of Health and Human Services. Tick-borne disease working group: 2018 report to congress [Internet]. Washington (DC); US Department of Health and Human Services: 2018 [updated 2018; cited 2019 Oct 10]. Available from: https://www.hhs.gov/sites/default/files/tbdwg-report-to-congress-2018.pdf
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009 Aug; 339(7): 332-336. Available from: https://www.jstor.org/stable/25672329?seq=1&cid=pdf-reference#references_tab_contents
23. National Library of Medicine. Unified Medical Language System (UMLS) [Internet]. Bethesda (MD): National Institutes of Health; 2019 [updated 2019 May 23; cited 2019 Feb 1]. Available from: https://www.nlm.nih.gov/research/umls/index.html
24. Goldfarb D, Sataloff RT. Lyme disease: A review for the otolaryngologist. Ear Nose Throat J. 1994 Nov; 73(11): 824-9.
25. Haney C, Nahata MC. Unique expression of chronic Lyme disease and Jarisch-Herxheimer reaction to doxycycline therapy in a young adult. BMJ Case Rep. 2016 Jul; doi: 10.1136/bcr-2013-009433.
26. Heir GM. Differentiation of orofacial pain related to Lyme disease from other dental and facial pain disorders. Dent Clin North Am. 1997; 41(2): 243-58.
27. Heir GM, Fein LA. Lyme disease awareness for the New Jersey dentist: A survey of orofacial and headache complaints associated with Lyme disease. J N J Dent Assoc. 1997 Dec; 69(1): 19, 21, 62-3, 69.
28. Heir GM, Fein LA. Lyme disease: Considerations for dentistry. J Orofac Pain. 1996 Win; 10(1): 74-86.
29. Emkey GR, Stone JH. A 46-year old woman with chin pain and a fainting spell. Arthritis Care Res. 2010 Mar; 62(3): 434-8.
30. Halperin JJ. Is neuroborresliosis a medical emergency? Neurocrit Care. 2006 Jun; 2006(4): 260-6.
31. Halperin JJ. Nervous system Lyme disease. Infect Dis Clin N Am. 2008 Jun; 22(2): 261-74.
32. Greer DM, Schaefer PW, Plotkin SR, et al. Case 11-2007: A 59-year old man with neck pain, weakness in the arms, and cranial-nerve palsies. N Engl J Med. 2007 Apr; 356(15): 1561-70.
33. Greenberg MR, Urquhart MC, Eygnor JK, et al. I can't move my face! A case of bilateral facial palsy. J Am Osteopath Assoc. 2013 Oct; 113(10): 788-90.
34. Glickstein L, Moore B, Bledsoe T, et al. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun. 2003 Oct; 71(10): 6051-3.