You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
As the severe acute respiratory syndrome 2 (SARS-COV-2) coronavirus disease 2019 (COVID-19) outbreak swept across the United States and led to the declaration of a public health emergency, dentistry began to critically evaluate infection control standards, aerosol mitigation strategies, and personal protective equipment policies to assess safety during the pandemic. Many professionals looked to entities such as the Centers for Disease Control and Prevention (CDC) for guidance. However, currently, not all states in the US claim to follow the CDC guidelines when it comes to alignment within their dental practice act and subsequent provisions of infection control standards.1
With the outbreak and subsequent severity of COVID-19, dental hygienists experienced fears around the role of patient care and its risk to their health, particularly with the production of aerosols generated during mechanized scaling procedures, or ultrasonic instrumentation of the root surface (known as root surface debridement),2 in addition to the concern many dental professionals have of perceived violations of infection protocols in the clinical setting.3
In turn, the concerns over transmission of infection via aerosols in the dental operatory have led to widespread modifications to dental patient care during the global pandemic of COVID-19. However, research has also begun to demonstrate the important role of preventive and periodontal therapy, and how this role may efficiently impact the prevention and control of the spread of COVID-19 disease in communities. Additionally, research has identified that dental professionals hold a profound understanding of infection control modalities, transmission rates, and preventive strategies to best protect themselves and the community during the COVID-19 crisis.4
This article aims to critically evaluate safety as it relates to treatment alterations, specifically during mechanized scaling, outlining the current research as it is known about aerosols generated during periodontal treatment, the subsequent fears of patient, and provider safety, particularly during the global health crisis of COVID-19, and the treatment modifications currently being instituted as aerosol mitigation strategies during periodontal treatment. Specifically, this article outlines S.A.F.E. therapy with ultrasonic instrumentation, critically evaluating 1) scaling, 2) aerosols, 3) fears, and 4) efficiency as related to optimized patient care during this current global health crisis.
Scaling
Prior to the COVID-19 pandemic, dental professionals across the United States readily generated aerosols through a variety of procedural modalities, including but not limited to procedures that use low- and high-speed handpieces, laser or electrosurgery units, ultrasonic scalers, air polishers, prophy angles, hand instrumentation, and air/water syringes.5 Despite the ready presence of various bloodborne and airborne diseases in the dental operatory, systematic reviews of the adherence to infection control guidelines in dentistry revealed that in some cases (such as donning gloves during procedures) there have been improvements and yet in other cases, many concerns with regards to infection control compliance exist, noting inadequate management of needlesticks as one problematic area.6In addition, training of staff on infection control standards and its subsequent documentation are requirements of a managed system in the dental practice.7
Following the nationwide shutdown due to the COVID-19 pandemic, many clinicians dramatically adjusted their clinical routines to better accommodate CDC guidelines. One of these adjustments was in the reduction of use or the complete elimination of mechanized scalers (sonic and ultrasonic scalers) for the preventive or active treatment of periodontal disease to reduce the risk of airborne acquisition of viral aerosols.
The following sections critically evaluate considerations with regards to the safe and efficient provision of preventive and periodontal therapy through mechanized scaling and, more specifically, ultrasonic scalers.
Equipment Considerations
Considered to be a potential reservoir for pathogenic microorganisms and subsequently the SARS-CoV-2 virus, dental equipment must continually be treated and managed to reduce the risk of spread of infection by vector.
Clean Dental Lines
After a period of non-use, dental equipment may require additional maintenance or repair beyond the standardized maintenance required. One area of particular concern is the ability for microorganism growth within the dental unit waterline (DUWL). Research has indicated that the extent of bacterial contamination notated in the DUWL is a serious issue in dentistry.8
Interestingly, studies have indicated that flushing the DUWL for 3 minutes had no impact on the contamination of water from controlled opportunistic bacteria such as P. aeruginosa, S. aureus, S. auricularis, P. fluorescens,and A. baumannii.9
The following steps are encouraged to safely and efficiently treat the DUWL with compliance: 1) flush water lines at the beginning of every day, 2) flush water lines between patients, 3) use water that meets regulatory standards, which may require pretreatment of the water, 4) routinely test the water in the DUWL according to manufacturer recommendations, 5) apply chemical debridements via chemical shock of the DUWL according to manufacturer recommendations, and 6) ensure ultrasonic filters are routinely replaced.9
It is imperative to note that the DUWL can serve as a significant source for infection of respiratory systems, and although there are limited published cases of said transmission, dental professionals are required to comply with DUWL maintenance standards to align with water safety regulations.10
Evaluate Vacuum Capacity
As recently as 2006, the Centers for Disease Control and Prevention issued guidelines around safe evacuation systems, encouraging suction lines in treatment rooms to be cleaned every day with an evacuation system cleaner to remove blood and debris, and a disinfectant that is compatible with the evacuation system should be run through the tubing.11 While the phenomenon of backflow has not been demonstrated in high-volume suction lines, it has been readily detected in low-volume suction lines, and as such, recommendations have been made to disinfect suction lines between patients.11
In addition, it is important to evaluate the power and flow of evacuation systems periodically to ensure there is sufficient static measurement of vacuum pressure, as a low static pressure results in higher risk of backflow.11 A timed water test is one way to efficiently test standard evacuation devices against various other evacuation devices (Figure 1).
Evaluate Pump Efficacy
With the introduction of multi-operatory high-volume evacuation units continually drawing on the pump to maximize evacuation, it is imperative to ensure that the current pump system or systems have the appropriate capabilities to distribute the workload evenly throughout the office. This even workload ensures a delivery of constant suction capabilities.12,13
As additional operatories begin utilizing high-volume evacuation systems, dental practice owners may consider increasing the size of the pump unit or upgrading to a dry vacuum in which flow and high negative pressures combined provide optimal support for the dental practice. Consulting with the manufacturer will help evaluate the efficacy of a pump system.12,13
Layered Protection
A well-documented approach to the safe delivery of aerosol-generating dental procedures involves a layered approach to patient and clinician protection. Specifically, the mitigation of risk via ultrasonic scaling has been well published, noting the safest strategies include: 1) appropriate patient selection, 2) preprocedural rinsing, 3) properly wearing personal protective equipment, 4) properly disinfecting areas of splash, splatter, and aerosols, 5) good ventilation and air circulation in the operatory, and 6) appropriate DUWL maintenance.14
Ultrasonic Use
Achieving a healing response in successful periodontal debridement is highly dependent on the thorough nature of instrumentation. Specifically, thoroughness is widely dependent upon the ability of instrumentation to contact the root surface, the efficacy of deposit removal, the efficiency of deposit removal, and the subsequent effect on the root surface, in tandem with the ability for the periodontal instrumentation of choice to adapt, angulate, and activate, while also acknowledging the importance of patient comfort and optimal clinical ergonomics.15
While successful debridement has been noted in both hand and ultrasonic instrumentation, it is well understood that ultrasonic instrumentation is superior in meeting the above criterion when compared with hand instrumentation alone. Ultrasonic instrumentation includes piezoelectric technologies in which energy is generated through a crystal transducer modifying the ultrasonic tip and causing a linear activation16 or magnetostrictive technologies in which energy is generated through metal stacks to an ultrasonic tip that utilizes oscillating motion.16,17 However, the prudent clinician must be mindful of the careful considerations that can dramatically impact the quality and safety of ultrasonic instrumentation, including tip selection, water flow, and power settings necessary for efficient and effective debridement.
Tip Selection
Ultrasonic tip selection is as selective of a process as hand instrument selection may be. The clinician must consider: 1) type of deposit, which will alter the selection of a standard or slim nature of the tip, 2) anatomy of hard tissue structures, which will guide the need for a straight or curved tip, and 3) gingival biotype as either thick or thin, which can determine the selected diameter of the tip.18
Secondly, ultrasonic instrumentation is best implemented in a staged instrumentation approach, beginning with initial debridement with a standard insert indicated for the gross removal of moderate-to-heavy calculus or stain at a higher power level, followed by debridement in which definitive removal of light deposits, biofilm, and endotoxins is completed with a slim or modified insert on a low power setting.18 In addition, ultrasonic instrumentation provides the benefits of lavage or the delivery of an irrigant into the subgingival space with the intention of providing cooling as well as cavitation and acoustic microstreaming, which are discussed in the section below.18
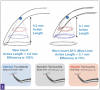
Tip wear greatly reduces efficiency: a new tip will deliver 100% efficiency, whereas a worn tip at 1 mm is now functioning at 75% efficiency and a worn tip at 2 mm will function at 50% of its original efficiency (Figure 2). Clinically, this may be observed as a lack of efficiency in removing calculus.18-20
Water Flow
Water flow is ideal as a coolant and provides accessory action of microstreaming, which is said to aid in biofilm disruption and/or removal by turbulent currents of water surrounding the tip and cavitation providing biofilm disruption and removal by shock waves from the implosion of bubbles.20 For magnetostrictive units, a rapid flow of drips out of the handpiece in addition to a fine mist demonstrates adequate water flow to ensure appropriate cooling.18 It should be noted that less water is required when using a piezoelectric unit or a magnetostrictive unit that has been manually tuned, and as such, a rapid flow of drips is not required for a piezoelectric unit.21 After ideal flow rate is established based on the mechanism of action of the ultrasonic unit, the power level setting should be adjusted depending on the task at hand. Specifically, regarding water flow as it relates to magnetostrictive units in a low-to-medium power setting, droplets at the tip are necessary to maximize cavitational activity. In higher power settings, the droplet is atomized and only a light mist is produced (Figure 3).20
Clinicians often increase water flow in an attempt to troubleshoot overheating challenges. Overheating can occur: 1) when the clinician does not fill the handpiece with water prior to insert insertion, 2) when the clinician is using an unserviceable insert, 3) if there is inadequate water pressure within the dental unit, or 4) if there is a clogged insert water port. It should be noted that in all of the cases mentioned, increasing water flow will not rectify the challenge, and consultation with the equipment manufacturer is warranted.18Additionally, it is noted that increasing water flow beyond necessary coolant measures significantly reduces tip vibrations, which may have a subsequent impact on efficiency of action of the ultrasonic tip.22
Power Setting
The power source within an ultrasonic scaler depends on its piezoelectric or magnetostrictive properties.17,20 Piezoelectric scalers receive their power via a crystal transducer that changes dimension, shape, and size, thus generating vibrations directed to the tip, permitting a linear stroke via two active lateral surfaces.16Magnetostrictive scalers receive their power via a stack of ferromagnetic metal strips that connect to a working tip delivering nodal points or regions and subsequently producing an elliptical stroke in which energy is evenly distributed on all surfaces of the tip.17,18,20
Frequency is defined as the number of cycles per second, measured in kilohertz, or the number of times per second the tip of the ultrasonic scaler moves back and forth during one orbital rotation, and it determines the active area of the tip. In most cases, frequency is predetermined within the unit and is not adjusted by the clinician.20
Amplitude affects the power level itself and further determines the stroke length, or how far the instrument tip moves during one cycle. A lower power setting will deliver shorter, less powerful strokes ideal for light deposit, biofilm, and endotoxin removal. A higher power setting will deliver longer, more powerful strokes and is appropriate for moderate-to-heavy calculus removal.18,20,21
In general, clinicians are instructed to select the lowest effective power setting for the task to be completed.18 In instances where tip wear is detected, the clinician is oftentimes tempted to increase the power setting to compensate and subsequently achieve greater efficiency with deposit removal. However, it should be noted that a worn tip with increased power setting will not resolve this challenge, as there is no longer ample tip length available to produce a high stroke amplitude.18,20
In addition to frequency and amplitude, ultrasonic vibrations are also impacted in points where no vibrations occur, also termed nodal points. Nodal points occur between 2.2 mm and 4.4 mm from the point of the ultrasonic insert. This is particularly concerning in consideration of tip adaptation, noting that if the terminal 2-3 mm of the tip are not in contact with the area to be treated, a nodal point may be reached, noting that the instrument is thus ineffective.23
Finally, aerosol production during power scaling is impacted by aspects such as the amplitude, shape of the tip, water delivery, and amount of water flow utilized during ultrasonic scaling. As such, it benefits the clinician to ensure that all aspects with regards to equipment considerations are evaluated prior to the integration of ultrasonic scaling in the dental operatory.18,20
Aerosols
Aerosols are loosely defined as airborne particulates containing liquid or volatile compounds containing living organisms or components released from living organisms.24 SARS-COV-2 is highly contagious and is said to be transmitted via aerosol droplet by direct contact with infected persons or the indirect contact of respiratory droplets expelled by an infected person.25
Comprehensive systematic reviews of current national and international research have yielded telling outcomes with regards to the generation of aerosols in dentistry. Notably, there are clear inconsistencies regarding the true definition of an aerosol-generating dental procedure (AGDP).26Findings include: 1) there is a lack of consistency in reporting specifically which procedures are truly considered AGDPs; 2) high-speed handpieces, air-water syringes, and mechanized scalers are considered high-risk AGDPs; 3) there is a lack of understanding the true risk of COVID-19 transmission with the use of slow-speed handpieces; 4) there is an agreement that the use of a rubber dam with high-volume evacuation has the ability to significantly reduce aerosol production; and 5) there is a lack of understanding which procedures constitute a low, moderate, or high risk of COVID-19 transmission.26
New data is now suggesting that while dental turbines in high-speed handpieces and mechanized scalers such as ultrasonic scalers with a built-in water spray are known to generate the greatest amount of aerosols in the dental operatory, their subsequent generation of aerosols is not equal.27 Conclusive research has noted that aerosol generation via an ultrasonic scaler is easily controlled, whereas controlling aerosols generated via a high-speed turbine varies greatly depending on the direction and scenario of air spray during the procedure.28
It should be noted that no in-office dental procedure is considered completely risk free, as dental patients are unable to don a facial barrier upon receiving dental care. When delivering care during a global health crisis in which herd immunity has yet to be established, it is the responsibility of dental practitioners to understand their role in reducing the risk of spread of infection. As such, it has become the discretion of the dental professional to integrate key elements in understanding best practices for the safe and efficient treatment of dental patients during this current health crisis.
COVID-19
The average diameter of the SARS-CoV-2 virus is 0.12 μm.29 Current research indicates that the SARS-CoV-2 virus is able to remain suspended in aerosols for up to 3 hours.25 It should be noted that there are current concerns regarding the lighter mass of the virus and the potential opportunity for traveling a further distance; this elevates the need for new research on the potential pathogenic nature of aerosols and subsequent threat to patients and professionals.30
The viral load needed for the transmission of SARS-CoV-2 is estimated at approximately 1000 viral particles per minute (vp/minute).31 To provide a reference, breathing expels approximately 20 vp/min, talking provides approximately 200 vp/min, and sneezing provides approximately 200 million vp/min.31
There is insufficient quantitative research regarding the size of particulate as well as the virulent concentration of aerosols generated during dental procedures.
High-Volume Evacuation
High-volume evacuation (HVE) offers the best solution for the control of aerosol particulates before they leave the mouth.31 When compared with low-volume evacuation (LVE) or saliva ejectors, HVE devices can reduce 90% to 98% of aerosols and prove to be an effective solution for containing and reducing aerosols, as well as subsequent risk of contamination in the operatory.32
HVE and LVE maintain the same static vacuum pressure; the difference in efficiency is due to the bore hole size of the device. A bore hole size of 8 mm at a minimum is required in order to efficiently remove aerosols at a rate consistent with an HVE device. It is worth noting that a device that fits an HVE port does not ensure the device itself is a true HVE device. Specifically, many readily available devices have the ability to attach to an HVE port, yet perform as an LVE device, providing greater efficiency with fluid pooling and control but not necessarily the efficient and high-level removal of aerosols.32
Additionally, HVE devices must be held approximately 6 mm to 15 mm away from the active handpiece tip in order to organize and remove aerosols generated during dental procedures efficiently. This is a noteworthy to consider when evaluating existing hands-free devices being utilized within the HVE port in the dental operatory.32
The two-handed approach to integrating HVE for the dental hygienist may provide a layer of challenges with regards to skill. As such, several HVE devices have been designed and are readily available for the clinician working in a two-handed clinical approach. The integration of any new device warrants ample time and training for the clinician to properly rehearse and execute the device with ease (Figure 4).27
Fears
The United States Occupational Safety and Health Administration (OSHA) had declared dental hygienists at high risk for the contraction of COVID-19.33The result: while dentists across the United States remained steadfast with high-speed handpiece in hand, many dental hygienists temporarily retired most aerosol-generating equipment within the hygiene operatory.
Primary elements that led to this conclusion were: 1) recommendations from governing bodies such as the state dental boards regarding the reduction of AGDPs during the pandemic, and 2) fears around the occupational risk associated with generating AGDPs to suspected and/or asymptomatic carriers of the SARS-CoV-2 virus.
Clinical Decision Making
During the nationwide shut down, dental professionals began to look to models such as the Safer Aerosol-Free Emergent Dentistry (SAFER) model, which suggested alternatives to aerosol-generating procedures during emergent dental care.34 While some aerosol-generating equipment had readily available alternative options, in other areas of clinical care, aspects of patient care were being fully omitted due to a lack of understanding around the risk-to-benefit ratio.
The result: aerosol-reduction urgency led dental hygienists to retire their ultrasonics and perform preventive and active therapy with hand scalers, while many clinicians also omitted rubber cup and air polishing entirely from the dental hygiene appointment. The question has been asked: in integrating alternatives or omitting procedural elements all together, does the ethical integrity of the hygiene appointment remain?
It was quickly identified that initiating high-level moral decision-making35 around the safe provision of dental care during this pandemic was difficult in the face of unreliable research, evidence, and guidance. In addition, current research indicates that COVID-19 rate among dental hygienists is significantly low, additionally noting that over 99% of dental hygienists report that their dental practices boosted infection control standards such as personal protective equipment, in strict alignment with infection control guidelines by the CDC.36This research notes that oral health care can be safely provided during a global health crisis under appropriate conditions.36
Moral Decision Making
Current research has now indicated that the presence of periodontitis increases the risk of dying after a COVID-19 infection by nearly nine times.37 Notably, periodontitis was associated with a higher risk of intensive care admission, a need for assisted ventilation, and subsequent death of COVID-19 patients, indicating that these individuals presented with increased blood levels of biomarkers that increased their risk of experiencing worse disease outcomes.37
As such, a comprehensive evaluation of preventive and periodontal therapy is critical, particularly during the global health crisis. Specifically, it is imperative for dental hygienists to understand the delicate balance within the risk-to-benefit ratio when it comes to clinical considerations during the COVID-19 pandemic.
Paradigm shifts in preventive and periodontal therapies have moved away from the classical scaling and root planing model intended to remove "infected" cementum to a modern approach of root-surface debridement with primarily ultrasonic systems to remove hard mineralized deposits.38,39 There are many contributory factors to this paradigm shift.
First, ultrasonic instrumentation provides additional benefits, reducing instrumentation time by upwards of 37% when compared to hand scaling alone.40 Secondly, research has identified that this paradigm shift improves biofilm management while reducing discomfort for the patient during and after treatment.41
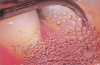
A likely reason for these observations is due to the superior efficiency ultrasonic instrumentation provides in readily accessing and subsequently treating anatomical considerations where hand instrumentation may not.42 Another consideration is the antimicrobial effect of ultrasonic treatment via the acoustic microstreaming, mechanical, irrigation, and cavitational activity of the tip, which is an important contributory factor for the clinical efficacy of the ultrasonic scaler (Figure 5).19
Efficiency
As research continues to unpack details regarding the role of oral disease in the contraction of COVID-19 disease, a critical evaluation of how mechanized scaling can efficiently reduce the length of dental appointment procedures and more predictably provide appropriate preventive and periodontal therapy is necessary.
Oral Health and COVID-19
Prior to the current global health crisis, it was well understood that good oral hygiene has been recognized as a means to prevent airway infections.43 As such, research began to postulate that ample oral hygiene may have a positive impact in reducing the spread of the SARS-CoV-2 virus.44
It is now believed that oral bacteria is a significant factor in facilitating co-infections in COVID-19.45 This facilitation may be due to the introduction of bacteremia, the subsequent inflammatory process46 associated with pathogenic bacteria, or the aggravation due to aspiration of pathogenic bacteria.47
Nevertheless, it is projected that oral hygiene routines across the United States have declined significantly in an era of social distancing.48As such, the work of the dental clinician in reducing bacterial loads and subsequent inflammatory conditions in the host has become impactful in the control of the COVID-19 pandemic. Therefore, the conversation around the provision of dental treatment during the pandemic moves beyond infection control strategies and extends into the importance of disease prevention and management.
Inflammatory Cytokines
The phenomenon of cytokine storm syndrome has led researchers to evaluate the role that inflammation may play in elevating risk of experiencing COVID-19. Broadly speaking, cytokine storm syndrome refers to an immune response in which there is a high release and subsequent concentration of inflammatory mediators such as interferons, interleukins, tumor-necrosis factors, chemokines, and other mediators.49,50
Of note, patients with COVID-19 present with reported elevated levels of specific inflammatory cytokines, particularly interleukin-6 (IL-6).51Moreover, elevated levels of IL-6 accurately predicted respiratory failure in individuals, elevating their risk to 22 times higher for respiratory complications.52
Another concern has been the ability for epithelium within the respiratory tract to become altered by periodontally-associated cytokines in which there is a direct promotion of infection via respiratory pathogens.53 During an active state of inflammation, the host releases leukotrienes, another inflammatory mediator. Leukotrienes have the ability to cause inflammation, including bronchoconstriction and airway obstruction, and subsequently increase cellular infiltration and cytokine release, including interleukins.54
Clinical Decision Making
The oral cavity is a known reservoir for respiratory pathogens, and research continues to hypothesize that improving oral health can decrease the severity and morbidity associated with COVID-19 disease.55 The pathophysiology of oral inflammation and rooted cytokine response has increased the need for recommending appropriate maintenance of oral hygiene in the COVID-19 era, as well as "red-flagging" patients with periodontitis as having an increased risk of the disease and adverse outcomes.48
As such, the improvement of oral health across the country must begin by critically aligning current patient care protocols with evidence-based dentistry, aiming to best mitigate widespread risk of COVID-19 disease through infection control and disease prevention strategies.
Summary
As dental practitioners across the United States continue to treat the community during the COVID-19 health crisis, it becomes imperative to continually evaluate current patient care strategies. While fears of safety and perceived risk of the contraction of COVID-19 disease continue to take an active part in driving clinical decision making for dental hygienists, the integration of new research gives providers the opportunity to continually clarify appropriate steps and strategies for the provision of evidence-based dentistry during a global health crisis.
Notably, the ability for the clinician to integrate modifications that best align with evidence-based dentistry while ensuring high-level safety during the COVID-19 pandemic has become increasingly critical. Within the current landscape of dentistry, the need for optimal infection control standards and the safe and efficient delivery of dental care to the community has never been greater.
All images provided with permission from Dentsply Sirona.
References
1. Licensing requirements. In: The Composite. 28th ed. Chicago, IL: American Association of Dental Boards. 2017: 68-69.
2. Ciantar M. Time to shift: from scaling and root planing to root surface debridement. Prim Dent J.2014;3(3):38-42. doi: 10.1308/205016814812736592.
3. Loch C, Kuan IBJ, Elsalem L, et al. COVID-19 and dental clinical practice: Students and clinical staff perceptions of health risks and educational impact. J Dent Educ. 2021;85(1):44-52.
4. Khader Y, Al Nsour M, Al-Batayneh OB, et al. Dentists' awareness, perception, and attitude regarding COVID-19 and infection control: cross-sectional study among Jordanian dentists. JMIR Public Health Surveill, 2020;6(2):e18798.
5. Hallier C, Williams DW, Potts AJC, Lewis MAO. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br Dent J. 2010;209(8):E14.
6. Gordon BL, Burke FJ, Bagg J, et al. Systematic review of adherence to infection control guidelines in dentistry. J Dent. 2001;29(8):509-516.
7. National Center for Immunization and Respiratory Diseases. Guidance for dental settings. Interim infection prevention and control guidance for dental settings during the COVID-19 response. Centers for Disease Control and Prevention web site. Updated December 4, 2020, https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html. Accessed March 20, 2021.
8. Alkhulaifi MM, Alotaibi DH, Alajlan H, Binshoail T. Assessment of nosocomial bacterial contamination in dental unit waterlines: Impact of flushing. Saudi Dent J.2020;32(2):68-73.
9. O'Donnell M J, Boyle MA, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol, 2011;6(10):1209-1226.
10. Pankhurst CL, Coulter WA. Do contaminated dental unit waterlines pose a risk of infection? J Dent. 2007;35(9):712-720.
11. Centers for Disease Control. Saliva ejector & backflow. Updated March 3, 2016. https://www.cdc.gov/oralhealth/infectioncontrol/faqs/saliva.html. Accessed March 01, 2021.
12. Elzein R, Bader B, Rammal A, et al. Legal liability facing COVID-19 in dentistry: Between malpractice and preventive recommendations. J Forensic Leg Med, 2021;78:102123. doi: 10.1016/j.jflm.2021.102123.
13.Milejczak CB. Optimum travel distance of dental aerosols in the dental hygiene practice. JDH. 2005; 81(4):20-21.
14. Lee YH, Auh QS. Strategies for prevention of coronavirus disease 2019 in the dental field. Oral Dis.2021;27(Suppl 3):740-741.
15. Paterson, M. The effect of instrumentation technique on the outcome of treatment for periodontitis. MSc(R) thesis. University of Glasgow. Submitted October 2020. http://theses.gla.ac.uk/81911/1/2020PatersonMichMScR.pdf. Accessed May 4, 2021.
16.Lewis J, Piezosurgery in dentistry and oral surgery. Veterinary Practice News. February 19, 2020. https://www.veterinarypracticenews.com/piezosurgery-in-dentistry-and-oral-surgery/. Accessed May 4, 2021.
17. Busslinger A, Lampe K, Beuchat, M, Lehmann B. A comparative in vitro study of a magnetostrictive and a piezoelectric ultrasonic scaling instrument. J Clinical Periodontol. 2001:28(7):642-649.
18. George MD, Donley TG, Preshaw PM. Ultrasonic Periodontal Debridement: Theory and Technique. Hoboken, NJ: Wiley-Blackwell; 2014.
19. Arabaci T, Ciçek Y, Canakçi CF. Sonic and ultrasonic scalers in periodontal treatment: a review. Int J Dent Hyg. 2007;5(1):2-12.
20. Gehrig JS, Houseman GA. Fundamentals of Periodontal Instrumentation. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000
21. Guignon AN. Understanding power scaling inserts and tips. RDH. March 1, 2012. https://www.rdhmag.com/infection-control/sterilization/article/16405902/understanding-power-scaling-inserts-and-tips. Accessed May 4, 2021.
22. Lea SC, Lightstone JJ, Landini G, Walmsley AD. The effect of water flow rate on ultrasonic scaler performance: in vitro evaluation. Perio. 2008;5(1):51-55.
23. Garlough D. Turning the heat down: Solutions for reducing chronic inflammation in our patients' bodies. RDH. March 14, 2017. https://www.rdhmag.com/patient-care/article/16409883/turning-the-heat-down-solutions-for-reducing-chronic-inflammation-in-our-patients-bodies. Accessed April 2, 2021,
24. Cox CS, Wathes CM. (Eds.). Bioaerosols Handbook. Boca Raton, FL: CRC Press; 2020.
25. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567.
26. Virdi MK, Durman K, Deacon S. The Debate: What are aerosol-generating procedures in dentistry? A rapid review [published online ahead of print, 2021 Jan 29]. JDR Clin Trans Res. 2021;2380084421989946. doi:10.1177/2380084421989946
27. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429-437. .
28. Kun-Szabó F, Gheorghita D, Ajtai T, et al. Aerosol generation and control in the dental operatory: An in vitro spectrometric study of typical clinical setups. PLoS One. 2021;16(2):e0246543. Published 2021 Feb 4. doi:10.1371/journal.pone.0246543
29. Neuman BW, Adair BD, Yoshioka C, et al. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J Virol. 2006;80(16):7918-7928.
30. Wu M, Chang YC. COVID-19 and its implications in dental care management against bioaerosol transmission. J Dent Sci. 2020;15(3):367-368.
31. Mupparapu M. Aerosol reduction urgency in post-COVID-19 dental practice. Quintessence Int. 2020;51(7):525-526.
32. Avasth,A. High volume evacuator (HVE) in reducing aerosol - an exploration worth by clinicians. J Dent Health Oral Disord Ther.2018;9(3):165-166.
33. Banakar M, Lankarani KB, Jafarpour D, et al. COVID-19 transmission risk and protective protocols in dentistry: a systematic review. BMC Oral Health. 2020;20(1):275.
34. Benzian H, Niederman R. A dental response to the COVID-19 Pandemic-Safer Aerosol-Free Emergent (SAFER) dentistry. Front Med (Lausanne).2020;7:520.
35. Coulthard P. Dentistry and coronavirus (COVID-19) - moral decision-making. Br Dent J. 2020;228(7):503-505.
36. Estrich CG, Gurenlian JR, Battrell A, et al. COVID-19 Prevalence and Related Practices among Dental Hygienists in the United States. J Dent Hyg. 2021;95(1):6-16.
37. Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID-19 infection: A case-control study [published online ahead of print, 2021 Feb 1]. J Clin Periodontol. 2021;10.1111/jcpe.13435. doi:10.1111/jcpe.13435
38. Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal medicine: 100 years of progress. J Dent Res. 2019;98(10):1053-1062.
39. Sahingur SE. Evolving Paradigms in the Pathogenesis and Management of Periodontitis. In Emerging Therapies in Periodontics. Cham, Switzerland: Springer, Cham:3-12.
40. Tunkel J, Heinecke A, Flemmig TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol.2002;29 Suppl 3:72-91.
41. Bastendorf KD, Strafela-Bastendorf N, Lussi A. Mechanical removal of the biofilm: is the curette still the gold standard? Monogr Oral Sci. 2021;29:105-118.
42. Begum N, Nagarathna DV, Vanasi M, et al. ‘Periodontal therapy simplified' - with the aid of technical advancements and minimally invasive procedures. International Journal of Innovative Science and Research Technology. 2018;3(9):582-587.
43. Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc. 2008;56(11):2124-2130.
44. Sampson V, Kamona N, Sampson A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br Dent J. 2020;228(12):971-975.
45. Patel J, Sampson V. The role of oral bacteria in COVID-19. Lancet Microbe. 2020;1(3):e105.
46. Sahni V, Gupta S. COVID-19 & Periodontitis: The cytokine connection. Med Hypotheses. 2020;144:109908. doi:10.1016/j.mehy.2020.109908.
47. Takahashi Y, Watanabe N, Kamio N, et al. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci. 2020;63(1):1-3.
48. Baptista AS, Prado IM, Perazzo MF, et al. Can children's oral hygiene and sleep routines be compromised during the COVID-19 pandemic?. Int J Paediatr Dent. 2021;31(1):12-19.
49. Gao YM, Xu G, Wang B, Liu B-C. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J Intern Med. 2021;289(2):147-161.
50. Ruscitti P, Berardicurti O, Iagnocco A, Giacomelli R. Cytokine storm syndrome in severe COVID-19. Autoimmun Rev. 2020;19(7):102562.
51. Sinha P, Matthay MA, Calfee CS. Is a "Cytokine Storm" Relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152-1154.
52. Xu J, Yang X, Huang C, et al. A Novel Risk-Stratification Models of the High-Flow Nasal Cannula Therapy in COVID-19 Patients With Hypoxemic Respiratory Failure. Front Med (Lausanne). 2020;7:607821. Published 2020 Dec 8.
53. Hayata M, Watanabe N, Tamura M, et al. The Periodontopathic Bacterium Fusobacterium nucleatum Induced Proinflammatory Cytokine Production by Human Respiratory Epithelial Cell Lines and in the Lower Respiratory Organs in Mice. Cell Physiol Biochem. 2019;53(1):49-61.
54. Sirois P. Leukotrienes: One step in our understanding of asthma. Respir Investig, 2019;57(2):97-110.
55. Botros N, Iyer P, Ojcius DM. Is there an association between oral health and severity of COVID-19 complications? Biomed J. 2020;43(4):325-327.