You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Dental implants were once considered uncommon in the United States (U.S.), however, are now considered customary and the standard of care for supporting dental restorations in edentulous areas. While the field of implant dentistry has demonstrated progress and increasing acceptance in recent decades, complications such as inflammatory peri-implant
disease, which can lead to failures, may occur.1-9 The prevalence of peri-implant diseases is controversial since the definition for peri-implantitis has changed numerous times in the past 10 years.10-15 Nonetheless, peri-implant disease is a frequently discussed topic of concern among clinicians and researchers.10-15 The prevalence of peri-implant inflammatory disease has been reported at 43% to 63.4% for mucositis and 18.8 to 22% for peri-implantitis.4-6 The variability in disease estimates may be influenced by an inconsistent criteria for diagnosing peri-implant disease, patient risk factors, and maintenance history.12,15
Even by conservative estimates, peri-implant disease is a current and future challenge for both the patient and oral health care professional.10,11,13,14 Existing evidence suggests clinicians will be required to help manage more patients with peri-implant disease, requiring more in-office maintenance related interventions.7,14,16 How dental professionals approach maintenance of dental implants becomes relevant to the long-term stability of tissues supporting dental implants.16-18
Peri-implant disease (based on clinical signs of inflammatory disease, such as bleeding on probing and/or suppuration and radiographic bone loss) is established and enhanced by several risk factors/indicators, including periodontal disease, diabetes, smoking, bruxism, residual cement, irregular oral hygiene maintenance programs, and poor plaque control skills (e.g. high plaque levels and microbial dysbiosis).1,7-9,13,14,17,19-24 Ferreira et al. conducted a longitudinal study of 212 patients followed for 10 years and discovered that those with high plaque levels were 14 times more likely to develop peri-implantitis.25
In addition to proper lifelong self-care, patients with implant-borne restorations require professional maintenance to safeguard their investment.16-20,26-30 According to the American Academy of Periodontology, many of the risk factors for peri-implant disease can be reduced through routine evaluations, early identification and intervention, and adherence to a structured maintenance program.1,31 In a five-year longitudinal study of over 200 subjects, Costa et al. reported that 44% of participants developed peri-implantitis if not in a maintenance program, while only 18% developed peri-implantitis if they adhered to a maintenance program.20 Professional implant maintenance programs include assessments such as bleeding upon probing (BOP) and suppuration, which are just two of the important clinical findings in detecting and monitoring peri-implant diseases.8,9,17,22,26,32 Routine gentle probing, at least once per year, has been identified to be part of the comprehensive oral exam for patients with dental implants.32 In addition, debridement around the implants includes devices and instruments compatible with implant surfaces.17,18,26 If scaling is necessary, caution should be used with metal instruments, as they may scratch the titanium implant surfaces.33-36 A 2012 systematic review evaluated the effects of different instruments on titanium implant surfaces and identified that non-metal instruments, rubber cup and air abrasives caused the least surface alteration to smooth and rough implant surfaces and maintained the implant surface integrity.33
Dental hygienists' implant assessment techniques, choice of instrumentation, recall protocols, and self-care recommendations are fundamental in the maintenance and prevention of peri-implant tissue diseases. There are approximately 185,000 licensed dental hygienists in the United States.37 Presently, dental implant maintenance is not a competency standard from the Committee on Dental Accreditation (CODA), the body that develops and implements education standards for dental hygiene programs.38 Although implant curriculum guidelines for dental hygiene programs were developed and released in 1995 by a scientific panel of experts from the International Congress of Oral Implantologists (ICOI), it remains unknown how widely the suggested guidelines have been adopted and implemented in dental hygiene programs and clinical practice.39 Other implant maintenance care guidelines exist, however if those are widely recognized or utilized is largely unknown.17,18,26,28,40 Research suggests that dental hygienists may not be adequately prepared to care for patients with dental implants during routine maintenance care appointments.41 Ward et al. surveyed 213 dental hygienists in the Southeast region of the U.S. and discovered only 12% had received didactic and clinical training on implant care during their dental hygiene education.41
Limited information is available on the implant care practices of dental hygienists in the U.S. Given the global concern regarding inflammatory peri-implant disease and the emphasis on patient and provider implant care, the purpose of this study was to explore U.S. dental hygienists' attitudes and practices regarding dental implant assessment and maintenance care, and their sources of implant-related knowledge.
Methods
This cross-sectional, quantitative, web-based study was approved by the University of California, San Francisco (UCSF) Institutional Review Board (IRB). After a review of survey methodology42,43 and review of publications on implant assessment and maintenance,11,16-21,26,28,31,32 a survey instrument was developed by study investigators. The survey was partially based on the framework designed by Ward et al., which was used by permission.41
The 34-item survey included topics regarding demographic characteristics, implant assessment practices and attitudes towards maintenance practices. Demographic and practice items included: current clinical dental hygiene status, year of graduation from an entry-level program, degree earned, practice description, years of clinical practice, average hours of patient care per week, percentage of patients with dental implants, and U.S. state of practice. Implant assessment and maintenance practice items included: methods and frequency of implant assessments, commonly used instruments for implant debridement and their relative efficacy, commonly recommended oral hygiene aids, and recall frequency for hypothetical patients with and without risk factors for peri-implant disease. Attitudinal items assessed respondent's perceived ability to remove plaque around implants as compared to natural teeth. One item asked about sources of implant-related knowledge.
Prior to finalizing the survey items, the survey was reviewed by two UCSF subject-matter experts to assess content validity and acceptability. The survey instrument was revised based on the feedback. In addition, the survey was pilot-tested with a convenience sample of 16 participants (eight UCSF Master of Science in Dental Hygiene students and eight UCSF School of Dentistry faculty members) for clarity and feasibility. Modifications to the survey were made based on the comments and results. A second pilot test was conducted with a convenience sample of 10 practicing clinical dental hygienists to assess clarity, feasibility, and accessibility of the items. The final survey was revised based on the feedback from both pilot tests.
Sample recruitment and data collection
The study population included dental hygienists who were members of the American Dental Hygienists' Association (ADHA). The ADHA Research Department randomly selected 10,000 participants by computer randomization from a database of approximately 35,000 member dental hygienists. The ADHA emailed a link to the web-based survey instrument (Qualtrics; Provo, UT). The survey included a welcome page explaining the study purpose and information to obtain informed consent. Following the initial survey distribution, two follow-up emails were sent approximately one week apart to encourage participation. Data was collected in February and March of 2017.
Data analysis
Data was gathered and evaluated using Qualtrics software. All responses were reported as frequency distributions. A 5-point Likert ordinal scale, ranging from "not effective at all" to "extremely effective," was used for many questions. Categories of "extremely effective" and "very effective" and the categories of "not effective" and "not effective at all" were dichotomized for analysis purposes to "not effective" and "effective." A 4-point Likert ordinal scale was also selected for some questions and ranged from "never" to "always". Categories of "never" and "rarely" were combined to "never/rarely."
Results
Of the 10,000 email surveys distributed, 270 emails bounced back, leaving a total of 9,730 in the sample that received a link to the questionnaire. A total of 2,033 dental hygienists opened the survey, however 15 were left blank, leaving 2,018 respondents (n=2,018) for a participation rate of 21%. Due to missing data and rounded values, not all numbers and percentages totaled 2,018 and 100%.
Demographic and practice characteristics
Most respondents (85%, n=1,708) reported they were currently practicing clinical dental hygiene, 98% of these respondents (n=1,668) reported they provided dental hygiene services to patients with dental implants. Of those practicing clinically, a majority (82%, n=1,213) estimated that between 10-30% of their patients have one or more implants. Over half of respondents (67%) reported working in a general dental practice setting. There was a balanced representation of respondents from all four geographical regions of the U.S. and similar representation from year of graduation groups (Table I).
Assessment/evaluation methods
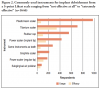
When queried about plaque removal, many respondents (44%) reported difficulty removing plaque around implants compared to natural teeth. When queried about the presence of bleeding and exudate, the majority of respondents (77%) reported that they always assess the gingiva and record bleeding and exudate. When queried about residual cement and probing, 34% reported never/rarely checking for residual cement, 31% never/rarely probed around implants, and 52% never/rarely checked for occlusion (Figure 1).
Instrumentation and perceived effectiveness
Plastic/resin scalers were the most commonly reported instrument used for debridement during routine implant care, selected by 60% of respondents. However, of those respondents, only 7% felt plastic/resin scalers were effective in implant debridement. Less than 5% of respondents reported using an air-polisher device for implant debridement; however, a majority (71%) of those who used air-polishers felt they were effective. Sixteen percent of respondents reported using the same instruments around implants as natural teeth (Figure 2). Five percent of respondents (n=70) indicated that they did not use any type of scaling instrument to debride around dental implants.
Maintenance recall
Items regarding recall frequency were asked using hypothetical patients with and without risk factors for peri-implant disease. For the patient with no risk factors for peri-implant disease, 58% of respondents (n=929) reported a six-month maintenance recall frequency in their practice, while 24% (n=392) indicated that recall frequency should be based on the individual patient needs. For the patient with risk factors of peri-implantitis (e.g. smoking, diabetes,
history of periodontitis), 58% of respondents (n=937) reported that the best maintenance recall frequency is every three months, while 21% (n=334) indicated that maintenance recall frequency should be based on individual need.
Recommended self-care aids for implants
When respondents were asked what type of oral hygiene aids they primarily recommend to patients for self-care for dental implants, responses included: oral irrigators (75%, n=1,208); floss products including monofilament or waxed (65%, n=1,044); tufted floss (59%,n=943); interdental/proxy brushes (55%, n=890); dental picks with synthetic rubber or silicone bristles (52%, n=837); specialty brushes such as a sulca-brush or end-tuft (41%, n=661); rubber tips (35%, n=557); wooden picks (12%, n=193); and air-floss devices (11%, n=174).
Sources of implant-related knowledge
The majority of respondents reported that their primary source of implant-related knowledge was continuing education courses, followed by professional interest magazines, and their employer/dentist (Table II).
Discussion
The aim of this study was to assess U.S. dental hygienists' practices and attitudes regarding implant assessment and maintenance care, as well as their sources of implant-related knowledge. Nearly all respondents currently practicing clinical dental hygiene provide care to patients with dental implants. Most respondents reported that 10-30% of the patients in their practice have one or more dental implants, which confirms the widespread acceptance of implant therapy in the U.S, and establishes the importance of dental hygienists' education and knowledge on this topic.
A majority of respondents routinely assess bleeding/exudate, mobility, plaque/calculus, and tissue color around dental implants. However, fewer respondents routinely check for residual cement, probe around an implant, or check occlusion. In this study, only 37% reported probing around implants during routine implant maintenance visits. This result differs with a study by Ward et al., in which 76% of respondents reported probing.41 A possible explanation for the differences in the study findings may have been a result of how the question was asked; Ward et al. asked a dichotomous yes/no question regarding probing around implants while this study assessed frequency of probing, "how often," at routine maintenance care appointments.
The difference in probing practices among dental hygienists may also stem from the controversy that exists among dental professionals, as probing may be thought to damage the peri-implant tissue, seal, and/or implant surface. However, to address that concern, Etter et al. concluded in their 2002 canine study that tissue trauma from clinical probing around implants is reversible, requiring four to five days for the epithelium to heal.44 Furthermore, Thierbach and Eger concluded that the presence of suppuration around an implant is a significant clinical parameter in determining the outcome of peri-implantitis treatment and reported that implants with suppuration frequently require surgical intervention for improved outcomes.45 A 10-year follow-up cohort study of 4,591 implants showed that suppuration and profuse bleeding was a meaningful observation in explaining marginal bone loss.46 Additionally, Salvi et al. suggests that tissue destruction around implants can be faster and more aggressive than around natural teeth,13 therefore frequent monitoring is advised. Although BOP around dental implants results in a higher rate of false-positive BOP rates than around teeth,46 diagnosing peri-implant disease and marginal bone loss solely by radiographic interpretation is problematic.1,47 Gentle probing around dental implants is a recommended clinical evaluation method by both the American Academy of Periodontology and the European Federation of Periodontology.1,8,9,31,32 Therefore, it is fundamental practice that dental professionals routinely monitor peri-implant soft tissues using a variety of techniques, including gentle probing, to detect early signs of biological complications for early clinical management, similar to natural teeth.
More than half of survey respondents never/rarely checked for residual cement around an implant. Assessment of residual cement is advisable, as residual cement may be associated with biologic complications.3,24 Given the popularity of cement-retained implant restorations and the high likelihood that a dental hygienist will encounter these restorations,48 evaluation of excess cement is recommended to reduce the associated inflammatory response and risk for peri-implantitis.3,24 These results suggest a necessity to further educate and reinforce the need to evaluate for residual cement during dental hygiene care appointments. In addition, it was found that more than half of the respondents never check implant occlusion, a similar finding to a previous implant survey study.41 Checking occlusal contacts can be helpful since implants are ankylosed and occlusal contacts can change. Compliance in the use of an occlusal guard, if the guard had been recommended, is advised. Additionally, evaluating the proximal contacts between implants and adjacent natural teeth is important since proximal contacts can open, resulting in food impaction and tissue irritation.49
The majority of respondents indicated using plastic/resin scalers for implant debridement. Similar results were reported from a previous survey of hygienists conducted in the U.S.41 Non-metal instruments have been identified as safe for implant debridement in the literature.33 Louropoulou et al., conducted a systematic review and evaluated the effects of different instruments on titanium implant surfaces and found that non-metal instruments, in addition to rubber cup and air abrasives, were most effective at maintaining implant surface integrity.33 This may explain why plastic scalers were reported by dental hygienists as the most commonly used instrument. However, almost all respondents (93%) who reported using plastic/resin scalers also indicated they are not effective instruments for implant debridement. An explanation for their perceived ineffectiveness may be the size of plastic scalers, as the bulky design of non-metal instruments may impose a significant challenge to access the submucosal regions around an implant.50
Very few respondents (5%) reported using air polishers for implant debridement, a finding consistent with Ward et al., where one-fifth of the participants reported air polishing use.41 The majority of the air polisher users (71%) in this study found the device to be very effective. The literature recommends the use of powered instruments such as air polishing devices in combination with low abrasive powders such as glycine or erythritol.18,33,51,52 While powered instruments should be considered as effective debridement methods for smooth and rough implant surfaces, there may be barriers to their implementation. The authors speculate
two practical barriers to air polishing usage include cost, since dental hygienists may not be key decision makers in practice equipment purchases, and lack of access to knowledge and/or training of air polishing technology. Removal of plaque biofilm and other hard deposits are basic principles to ensure implant longevity. In daily clinical practice, plaque may be the more frequent biological occurrence than calculus in routine implant maintenance. The traditional approach to scaling first, as with natural teeth, may not be the logical sequence for implant debridement. Air polishing or use of a rubber cup are suggested as preferred methods for biofilm management.33 If scaling is required, instruments that are effective and safe should be used. Additionally, emerging research shows that scratching as a result of instrumentation causes disruption of the titanium structure and oxide layer, which may lead to future inflammatory complications.53-55 Results from this study indicate that further studies on dental hygienists' perceived barriers and education related to implant debridement and instrumentation are needed.
Oral irrigation devices, followed by floss and tufted floss were the most common oral hygiene aids recommended to patients with dental implants. In some studies however, floss has shown to be a possible risk factor to supporting implant tissues, as flossing fibers may get trapped on the roughened implant surfaces.56-57 Despite the importance of effective plaque control for implant health, there is a lack of published research on the effects or benefits of powered oral irrigators, floss, and interdental brushes specifically around implants. Louropoulou et al. completed a systematic review on various self-performed mechanical oral hygiene aids and found that, while powered toothbrushes are beneficial for plaque removal, there is limited evidence demonstrating that powered toothbrushes are superior to manual toothbrushes.58 In addition, robust studies indicating the benefits of one interproximal cleaning device over another, was also lacking.58 Bidra et al. published clinical practice guidelines for implant-borne restorations including at-home maintenance specifications, however the strength of these recommendations was low due to the limited evidence available.18 Given the importance of daily mechanical plaque control on implant longevity, further research is recommended to identify optimal self-care oral hygiene aids for patients with dental implants.
The majority of respondents recommend a six-month recall frequency for individuals with no risk factors for developing peri-implant disease, which is similar to other published recommendations for patients at minimal risk.16,18,31 Many respondents indicated that a three-month recall frequency was recommended for those with risk factors, which is also consistent with recall frequencies recommended in the literature.17,31 Evidence-based recall frequency guidelines for patients with implants are not definitive; however, during supportive periodontal therapy, it is recommended that peri-implant tissues should be re-evaluated at each visit and recall frequencies should be tailored to the individual need of the patient.17,31 Dental hygienists should use a combination of knowledge, clinical judgment, experience, and patient's individual risks when considering recall frequency for patients with dental implants.59
Continuing education was the primary source of implant-related knowledge, followed by professional interest magazines, and their employer/dentist. Since one-third of respondents reported that they did not receive or had limited information about dental implants in their educational program, it was not surprising that continuing education was the most common source of implant-related knowledge. With regards to professional interest or industry magazines as the second most frequent source of dental implant knowledge cited, it should be noted that these publications may not be as scholarly or evidence-based as peer-reviewed journals. Results from this study showed a wide-range of practice patterns among dental hygienists. It is recommended that dental hygienists seek courses and publications that emphasize current scientific evidence to guide clinical practice decision making for patients with dental implants. Respondents reported both their employer/dentist and dental hygiene program as sources of implant knowledge. Future studies are needed to investigate implant-related knowledge, attitudes, and practices of U.S. dentists, and assess the dental implant curricula of dental hygiene educational programs in the U.S.
This study had limitations. Although there were over 2,000 respondents, the response rate was 21% from the random sample of 10,000. One explanation could be that ADHA members are inundated with requests to participate in online surveys and therefore fatigued to email survey requests. Results could also be affected by sampling bias, as all respondents are members of the ADHA and may be fundamentally different in their clinical practice behaviors than non-members. The findings could also be affected by response bias, as those who responded may have a greater interest in the topic than non-respondents. In addition, despite rigorous pilot testing, there were limitations to the survey items. The choices of instruments for debridement was not exhaustive. Furthermore, items regarding frequency and methods for taking radiographs was not assessed, which could have provided additional information on hygienists' practices. However, to the best of the authors' knowledge, this was the first study to explore implant care trends of dental hygienists throughout the U.S. and can serve as a resource for future studies of a larger population to validate the reported findings.
Conclusion
Early detection and prevention of peri-implant diseases are critical for dental implant health and longevity. While a majority of dental hygienists in clinical practice provide care to patients with dental implants, they demonstrate a wide range of assessment and maintenance practices. As the science of implantology advances, dental hygienists need to have current and comprehensive knowledge of evidence-based recommendations related to implant maintenance. Findings from this study highlight the need for implementing an evidence-based dental implant care curriculum in dental hygiene programs and continuing education settings as a means to increase the consistency and effectiveness of dental implant care and potentially decrease the prevalence of peri-implant diseases.
Disclosure
This study was supported by a research grant from the American Dental Hygienists' Association Institute of Oral Health (#17-01). Ivy H. Zellmer, RDH, MS, is a clinical professor; Elizabeth T. Couch, RDH, MS, is an assistant adjunct professor and project policy analyst; Lisa Berens, DDS, MPH, is a professor; Donald A. Curtis, DMD, is a professor; all in the Department of Preventive and Restorative Dental Sciences, University of California San Francisco, School of Dentistry, San Francisco, CA. Corresponding author: Ivy H. Zellmer, RDH, MS: ivy.zellmer@ucsf.edu
References
1. American Academy of Periodontology. Position paper. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013 Apr;84(4):436-43.
2. Daubert DM, Weinstein BF, Bordin S, et al. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015 Mar;86(3):337-47.
3. Renvert S, Quirynen M. Risk indicators for peri-implantitis. a narrative review. Clin Oral Implants Res. 2015 Sept;26 Suppl.11:15-44.
4. Atieh MA, Alsabeeha NH, Faggion Jr CM, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013 Nov;84(11):1586-98.
5. Derks J, Tomasi C. Peri-implant health and disease: a systematic review of current epidemiology. J Clin Periodontol. 2015 Apr;42 Suppl. 16:158-71.
6. Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017 Jul;62:1-12.
7. Dreyer H, Grischke J, Tiede C, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodont Res. 2018 Oct;53(5):657-81.
8. Heitz-Mayfield LJ, Salvi GE. Peri-implant mucositis. J Periodontol. 2018 Jun;89 Suppl. 1:S257-66.
9. Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Periodontol. 2018 Jun;89 Suppl. 1:S267-90.
10. Tarnow DP. Increasing prevalence of peri-implantitis: how will we manage? J Dent Res. 2016 Jan;95(1):7-8.
11. Papathanasiou E, Finkelman M, Hanley J, et al. Prevalence, etiology and treatment of peri-implant mucositis and peri-implantitis: a survey of periodontists in the United States. J Periodontol. 2016 May;87(5):493-501.
12. Buser D, Sennerby L, De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017 Feb;73(1):7-21.
13. Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017;96(1):31-7.
14. Berglundh T, Jepsen S, Standlinger B, et al. Peri-implantitis and its prevention. Clin Oral Implants Res. 2019 Feb;30(2):150-55.
15. Muñoz V, Duque A, Giraldo A, et al. Prevalence of peri-implant disease according to periodontal probing depth and bleeding on probing: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018 Jul/Aug;33(4):e 89-105.
16. Monje A, Aranda L, Diaz KT, et al. Impact of maintenance therapy for the prevention of peri-implant diseases: a systematic review and meta-analysis. J Dent Res. 2016 Apr;95:372-9.
17. Armitage GC, Xenoudi P. Post-treatment supportive care for the natural dentition and dental implants. Periodontology 2000. 2016 Jun;71:164-84.
18. Bidra AS, Daubert DM, Garcia LT et al. Clinical practice guidelines for recall and maintenance of patients with tooth-borne and implant-borne dental restorations. J Dent Hyg. 2016 Feb;90(1):60-9.
19. Anner R, Grossmann Y, Anner Y, et al. Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival: a long-term retrospective evaluation of patients followed for up to 10 years. Implant Dent. 2010 Feb;19(1):57-64.
20. Costa FO, Takenaka-Martinez S, Cota LO, et al. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012 Feb;39(2):173-81.
21. Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35 Suppl 8:292-304.
22. Kröger A, Hülsmann C, Fickl S, et al. The severity of human peri-implantitis lesions correlates with the level of submucosal microbial dysbiosis. J Clin Periodontol. 2018 Dec;45(12):1498-1509.
23. Stanford CM, Brand RA. Toward an understanding of implant occlusion and strain adaptive bone remodeling and remodeling. J Prosthet Dent. 1999 May;81:553-61.
24. Staubli N, Walter C, Schmidt JC, et al. Excess cement and the risk of peri-implant disease - a systematic review. Clin Oral Implants Res. 2017 Oct;28(10):1278-90. 25. Ferreira S, Silva G, Cortelli J, et al. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006 Dec;33:929-35.
26. Todescan S, Lavigne S, Kelekis-Cholakis A. Guidance for the maintenance care of dental implants: clinical review. J Can Dent Assoc. 2012;78:c107.
27. Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017 Oct;88(10):1030-41.
28. Koumkian JH, Kerver J, Smith RA. Hygiene maintenance of dental implants. J Calif Dent Assoc. 1990 Sep;18(9):29-33.
29. Gay IC, Tran DT, Weltman R, et al. Role of supportive maintenance therapy on implant survival: a university-based 17 years retrospective analysis. Int J Dent Hyg. 2016 Nov;14:267-71.
30. Lang NP, Berglundh T, Heitz-Mayfield LJ, et al. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxiollofac Implants. 2004;19 Suppl:150-4.
31. American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003 Sep;74(9):1395-1401.
32. Renvert S, Persson GR, Pirih FQ, et al. Peri-implant health, peri-implant mucositis, and peri-implantitis; case definitions and diagnostic considerations. J Clin Periodontol. 2018 Jun;45(Suppl. 20):S278-85.
33. Louropoulou A, Slot DE, van der Weijden F. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin. Oral Implants Res. 2012 Jun;23(6):643-58.
34. Kister F, Specht O, Warkentin M, et al. Peri-implantitis cleaning instrumentation influences the integrity of photoactive nanocoatings. Dent Mater. 2017 Feb;33(2):e69-e78.
35. Hasturk H, Nguyen DH, Sherzai H, et al. Comparison of the impact of scaler material composition on polished titanium implant abutment surfaces. J Dent Hyg. 2013 Aug;87(4):200-11.
36. Larsen OI, Enersen M, Kristoffersen AK, et al. Antimicrobial effects of three different treatment modalities on dental implant surfaces. J Oral Implantol. 2017 Dec;43(6):429-36.
37. American Dental Hygienists' Association. Dental hygiene diagnosis white paper [Internet]. Chicago (IL): American Dental Hygienists' Association; 2015 Sept [cited 2016 Dec 11]. Available from: https://www.adha.org/resources-docs/7111_Dental_Hygiene_Diagnosis_
Position_Paper.pdf
38. Commission on Dental Accreditation. Accreditation standards for dental hygiene education programs [Internet]. Chicago (IL): American Dental Association; 2013 January [cited 2019 Nov 19]. Available from: https://www.ada.org/~/media/CODA/Files/dental_hygiene_standards.pdf?la=en
39. Gurenlian JR, Meffert RM, Judt KM. Curriculum guidelines in implant dentistry for dental hygiene programs. Implant Dent. 1995 Fall;4(3):162-4.
40. Orton GS, Steele DL, Wolinsky LE. Dental professional's role in monitoring and maintenance of tissue-integrated prostheses. Int J Oral Maxillofac Implants. 1989 Winter;4(4):305-10.
41. Ward ST, Czuszak CA, Thompson AL, et al. Assessment and maintenance of dental implants: clinical and knowledge-seeking practices of dental hygienists. J Dent Hyg. 2012 Spring;86(2):104-10.
42. Richardson, J. Design and conduct a survey. Complement Ther Med. 2005 Mar;13(1):47-53.
43. Smith S. Introduction to survey research. [Internet]. Provo (UT): Qualtrics; 2017 [cited 2017 Feb 2]. Available from: https://www.qualtrics.com/support/research-resources/introduction-survey-research/
44. Etter TH, Håkanson I, Lang NP, et al. Healing after standardized clinical probing of the peri-implant soft tissue seal: a histomorphometric study in dogs. Clinical Oral Implants Res. 2002 Dec;13(6):571-80.
45. Thierbach R, Eger T. Clinical outcome of a nonsurgical and surgical treatment protocol in difference types of peri-implantitis: a case series. Quintessence Int. 2013 Feb;44(2):137-48.
46. French D, Cochran DL, Ofec R. Retrospective cohort study of 4,951 Straumann implants placed in 2,060 patients in private practice with up to 10-year followup: the relationship between crestal bone level and soft tissue condition. J Oral Maxillofac Implants. 2016 Nov-Dec;31(6):e168-78.
47. Walton TR, Layton DM. Intra- and inter-examiner agreement when assessing radiographic implant bone levels: differences related to brightness, accuracy, participant demographics and implant characteristics. Clin Oral Impl Res. 2018 Jul;29(7):756-71.
48. The National Dental Practice-Based Research Network. Quick roll results: cement-retained versus screw-retained implant restorations [Internet]. Birmingham (AL): National Dental Practice-Based Research Network; May 2017 [cited 2017 May 26]. Available from: http://files.constantcontact.com/c7da0d4c001/5fed8d69-9f01-465b-92f1-62e070fdca42.pdf
49. Fu JH, Hsu YT, Wang HL. Identifying occlusal overload and how to deal with it to avoid marginal bone loss around implants. Eur J Oral Implantol. 2012;5 Suppl:S91-103.
50. Goh EXJ, Lim LP. Implant maintenance for the prevention of biological complications: are you ready for the next challenge? J Invest Clin Dent. 2017 Nov;8(4):e12251.
51. Lupi SM, Granati M, Butera A, et al. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: A six month randomized clinical trial. Int J Dent Hyg. 2017 Nov;15(4):287-94.
52. De Siena F, Corbella S, Taschieri S, et al. Adjunctive glycine powder air-polishing for the treatment of peri-implant mucositis: An observational clinical trial. Int J Dent Hyg. 2015 Aug;13(3):170-6.
53. Mouhyi J, Dohan Ehrenfest DM, Albrektsson T. The peri-implantitis: implant surfaces, microstructure, and physicochemical aspects. Clin Implant Dent Relat Res. 2012 Apr;14(2):170-83.
54. Pettersson M, Kelk P, Belibasakis GN, et al. Titanium ions form particles that activate and execute interleukin-1b release from lipopolysaccharide-primed macrophages. J Periodontal Res. 2017 Feb;52(1):21-32.
55. Safioto LM, Kotsakis GA, Pozhitkov AE, et al. Increased levels of dissolved titanium are associated with peri-implantitis: a cross-sectional study. J Periodontol. 2017 May;88(5):436-42.
56. van Velzen FJ, Lang NP, Schulten EA, et al. Dental floss as a possible risk for the development of peri-implant disease: an observational study of 10 cases. Clin Oral Implants Res. 2016 May;27(5):618-21.
57. Montevecchi M, De Blasi V, Checchi L. Is implant flossing a risk-free procedure? A case report with a 6-year follow-up. Int J Oral Maxillofac Implants. 2016 May-June;31(3);e79-83.
58. Louropoulou A, Slot DE, van der Weijden GA. Mechanical self-performed oral hygiene of implant supported restorations: a systematic review. J Evid Based Dent Pract. 2014 Jun;14 Suppl:60-9.
59. Curtis DA, Lin GH, Fishman A, et al. Patient-centered risk assessment in implant treatment planning. Int J Oral Maxillofac Implants. 2019 Mar/Apr;34(2):506-20.