You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Disclosure: “Subperiosteal Minimally Invasive Aesthetic Ridge Augmentation Technique” and “S.M.A.R.T.” are trademarks of the author. The method and its associated devices are the subject of one or more pending patent applications.
The use of dental implants has become increasingly widespread and, along with it, the frequency of complications and unexpected outcomes has risen. In particular, iatrogenic sequelae from failed implant and bone-augmentation procedures in the esthetic zone pose a significant challenge because of the often catastrophic nature of the resulting gingival-alveolar defects. In the presence of a revealing smile, treatment of these complications represents a high-risk proposition for clinicians, which is compounded by potential medico-legal implications. The most difficult endeavor in these situations is the recreation of an ideal gingival architecture, particularly when dental implants are involved.
Alveolar ridge defects have traditionally been treated with surgical techniques that involve the reflection of a mucoperiosteal flap. Because these defects often require significant volume augmentation, a greater degree of flap reflection and advancement is needed to achieve adequate co-aptation. The risk of complications is also increased, including incomplete wound closure and soft-tissue dehiscences, which could lead to exposure of the membrane or graft material, infections, and compromised hard- and soft-tissue outcomes.1
An evaluation of the predictability of bone-augmentation procedures in the esthetic zone must take into account the resulting peri-implant soft-tissue architecture. This factor becomes a crucial concern in high-risk scenarios when improved esthetic outcomes are required and minimally invasive surgical techniques need to be considered.2 Additionally, the use of combined therapies, including orthodontic forced eruption and distraction osteogenesis, may provide more predictable alternatives to vertical bone augmentation while preserving the soft-tissue architecture.3,4
The purpose of this article is to demonstrate, through the presentation of a case report, the use of an interdisciplinary approach that included forced eruption and a novel subperiosteal minimally invasive (a)esthetic ridge-augmentation technique (SMART) for the treatment of a complex iatrogenic gingival-alveolar defect.
Case Presentation
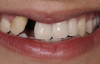
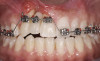
A healthy 20-year-old woman presented to the author’s office requesting treatment for a large defect in the area of missing tooth No. 7. She exhibited a high smile line that revealed a clearly visible deformity, associated with pain and sensitivity on teeth Nos. 6 and 8 and in the area of tooth No. 7 (Figure 1). Although the patient wore a modified Essix retainer, the defect was still visible because of the magnitude of tissue loss and the revealing nature of her smile.
The patient reported previous comprehensive orthodontic therapy, part of which included the creation of adequate space for replacement of the congenitally missing maxillary right lateral incisor. After completion of orthodontic therapy, implant placement and bone grafting were performed in the area of No. 7. Unfortunately, both procedures failed, resulting in a large hard- and soft-tissue defect. A subsequent attempt to perform bone augmentation was also unsuccessful and, instead, resulted in a larger deficit and increased recession on teeth Nos. 6 and 8. After a recommendation to attempt a third bone graft, the patient decided to seek alternative options.
Clinical Examination
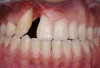
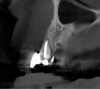
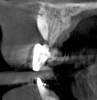
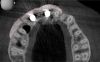
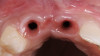
The intraoral examination revealed a substantial deficit of alveolar bone and gingival tissues in the maxillary right lateral incisor area. The ridge defect exhibited vertical and horizontal components, which were associated with a severe loss of clinical attachment on both the mesial aspect of tooth No. 6 and the distal aspect of tooth No. 8. Although probing depths were within normal limits, minimal keratinized gingiva was present and the soft tissues were acutely inflamed. Plaque removal was difficult because of the soft-tissue defect, gingival-margin location, and irregular soft-tissue architecture. Additionally, bone sequestration could be observed through the labial mucosa (Figure 2).
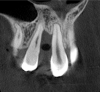
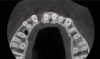
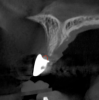
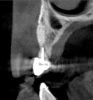
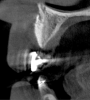
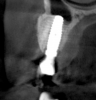
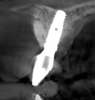
Tomographic images revealed a large tridimensional defect, with vertical and horizontal loss of bone extending to the apical third of teeth Nos. 6 and 8. Additionally, a buccal bone dehiscence was evident on tooth No. 5, and thin labial plates secondary to the orthodontic movement were present in several areas (Figure 3 and Figure 4).
Rationale for Implant Therapy in the Esthetic Zone
Establishing esthetic and restorative objectives is essential in treatment planning for complex defects in the maxillary anterior region. In the present case, teeth Nos. 6 and 8 required extraction and replacement with implants due to their poor prognosis. The patient’s high smile line and esthetic demands precluded the use of any prosthesis incorporating pink restorative materials.
Esthetic outcomes in implant therapy are highly dependent on the architecture of the peri-implant soft tissues.5 Additionally, the esthetic predictability of immediate postextraction implant placement has been well-documented.6-13 Lee and coworkers reported minimal changes in the thickness of the labial plate 6 months after treatment,12 and Chu et al similarly reported negligible remodeling of labial soft-tissue profiles.14
Although different protocols have been advocated, recent publications show consensus on the importance of clinician experience and adequate site selection in achieving predictable esthetic outcomes with immediate postextraction flapless implant placement and provisionalization.13,15,16 Therefore, a rationale for the predictable treatment of severe gingival-alveolar defects in the esthetic zone may require development of compromised sites into ideal sites before implant placement.
In the present case, site development of Nos. 6 and 8 with predictable soft-tissue esthetics would be a complex endeavor requiring the use of interdisciplinary therapy. Whether subsequent immediate implant placement and provisionalization would result in absolute preservation of the hard and soft tissues is less relevant than a comparison between the esthetic outcomes of this approach versus those achieved with traditional flap-based surgical augmentations.
Esthetic Predictability of Bone-Grafting Procedures
Bone-grafting procedures have been shown to be efficacious in providing adequate bone volumes for dental implant placement.4,17-19 However, the effect of these procedures on the peri-implant soft-tissue profiles is an important consideration in the esthetic zone. Several authors have reported that bone augmentation, guided bone regeneration (GBR), and flap-based techniques exhibit an increased risk of complications and compromised peri-implant esthetics, frequently resulting in sequelae ranging from scar formation and gingival defects to recession and deficient papillae, particularly in thin-biotype scenarios.2,20-22
In addition, the patient in this case report exhibited a large iatrogenic defect with a substantial vertical component, and, therefore, the predictability of vertical bone-augmentation procedures needed to be considered. In the results of an initial study, Merli and coworkers reported a 54% total complication rate in vertical augmentation procedures comparing the use of autogenous bone grafts with resorbable membranes and titanium plates versus titanium-reinforced barriers.23 In a subsequent study of vertical GBR outcomes using resorbable and nonresorbable membranes, Merli reported a total complication rate of 41%.24 The complications included membrane exposure, infections, and loss of the graft. Both studies were limited to posterior sites, and all surgical procedures were performed by a highly skilled clinician with more than 20 years of experience with dental implant surgery.
Because of the high-risk scenario in the present case, based on the iatrogenic nature and complexity of the defect, its location in the anterior maxilla, and the revealing smile line, traditional surgical bone-augmentation procedures (horizontal or vertical) were likely to present an unacceptable rate of complications and lack of esthetic predictability. Therefore, they were not considered as options for resolving the clinical challenges of this case.
Orthodontic Forced Eruption
Bone remodeling associated with orthodontic movement follows the basic principles described by Reitan. When a tooth is moved in a certain direction, bone resorption occurs on the side where pressure is applied to the periodontal ligament, while bone apposition is stimulated by the tension generated on the opposite side.25 In 1973, Brown reported the effect of orthodontic therapy on periodontal defects and the potential for changes in the hard- and soft-tissue architecture.26 In a classic report, Ingber described the use of orthodontic forced eruption and the resulting bone remodeling to treat periodontal infrabony defects.27 Pontoriero et al demonstrated how this technique could also be used to alter interproximal crest levels.28 Ingber subsequently reported on the benefits of soft-tissue remodeling associated with forced eruption in the treatment of cosmetic soft-tissue deformities.29 Salama and coworkers later described the use of forced eruption in hopeless teeth and how the resulting orthodontic extrusive remodeling of hard and soft tissues could be used to develop extraction-site defects before implant placement.30
Due to its predictability in achieving bone remodeling and developing alveolar ridge height while preserving or improving soft-tissue architecture, forced eruption was included as part of the interdisciplinary approach to treat the patient in the present case (Figure 5). Only stretching forces are applied to the periodontal ligament during the forced-eruption movement. This provides the stimulus for bone apposition within the socket walls. Generally, the orthodontic movement should be controlled so the root is extruded without impinging on the socket walls. In the presence of periodontal health, bone apposition and gingival remodeling will result in coronal proliferation of the attachment apparatus.29,30
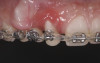
Occlusal management during the forced-eruption process is essential to avoid premature contacts that may displace the teeth labially and cause resorption of the buccal plate. The author recommends providing 2.5 mm of occlusal clearance at the start of forced eruption and performing weekly occlusal adjustments to re-establish this distance through the duration of the forced eruption. The rate of extrusion may vary, so patients should be instructed to contact the office for ad hoc adjustments at any point where contact with the opposing dentition may be perceived. Additionally, supracrestal fiberotomy may be performed to sever the periodontal ligament fibers and control the coronal migration of the attachment apparatus.28 In the present case, supracrestal fiberotomies were performed biweekly on the mesial half of tooth No. 8 and the distal half of tooth No. 6.
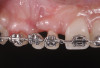
Successful outcomes with forced eruption require the establishment of treatment endpoints, which may include overcompensation beyond the desired soft- and hard-tissue changes. For the present case, achieving ideal gingival-alveolar socket architecture required force-erupting tooth No. 6 to the level of the apical third and tooth No. 8 beyond the confines of its alveolus. As the gingival sulcus is everted through the eruption process, a gingival red patch corresponding to the nonkeratinized sulcular epithelium may appear, as shown around tooth No. 8 in Figure 8 and Figure 9. This tissue will develop into keratinized gingiva when exposed to the oral environment.31,32 Additionally, when forced eruption of this magnitude is performed, there may be a tendency for lingual displacement that needs to be addressed with root-torquing orthodontic auxiliaries (Figure 6 and Figure 7).
When forced eruption is completed, the teeth should be splinted for a 3-month stabilization period,30 which will allow mineralization of osteoid tissue and settling of the gingival remodeling process. The degree of forced eruption in the present case was such that extreme mobility precluded the use of a provisional restoration. Instead, a metal-reinforced direct composite splint extending from teeth Nos. 6 to 8 was fabricated in situ (Figure 8 and Figure 9). When compared with the preoperative condition (Figure 2), Figure 8 and Figure 9 depict the treatment progression with forced eruption. The restoration of adequate alveolar height was achieved while enhancing the soft-tissue architecture, and the everted sulcular epithelium on tooth No. 8 proceeded to develop keratinization. However, the pre-existing defect still manifested itself in the form of a residual cleft.
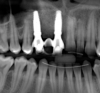
After 3 months of post-orthodontic stabilization, a cone-beam computed tomography scan was taken to re-evaluate the results and plan the future treatment sequence. Tomographic images clearly showed vertical gains in alveolar height, including in the edentulous area corresponding to tooth No. 7 (Figure 10 through Figure 12). The apex of tooth No. 8 was forced-erupted beyond its socket and could be visibly located within the soft tissue.30,33
Minimally Invasive Bone Grafting
The effect of complications secondary to traditional bone-augmentation procedures on the peri-implant soft-tissue architecture is an important consideration. Evidence suggests that flap-based surgical techniques, such as bone augmentation and GBR, may have a deleterious effect on implant esthetics.2,20-22 Minimally invasive procedures offer the potential to decrease postoperative complications and morbidity. Although a variety of tunneling techniques with particulate bone grafting have been attempted, they have not been widely accepted in clinical practice.34-39 Recent interest has focused mainly on soft-tissue tunneling applications.40-47
The author recently published a case series reporting the use of the SMART minimally invasive bone-grafting method. Bone augmentation was achieved in 60 sites within five treatment categories, with a maximum 30-month follow-up and human histology. The results demonstrated predictable and consistent bone augmentation, with a reduction in morbidity and complications.48 The mean horizontal augmentation in edentulous ridges was 6.47 mm with the SMART approach, while the average gain in ridge width for all treatment categories was 5.11 mm. These results compare favorably with previously reported horizontal augmentation outcomes using traditional GBR techniques.49-54
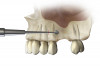
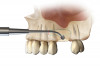
For the present case, the SMART method was used to achieve horizontal bone augmentation while preserving the soft-tissue profiles developed using forced eruption. Flap elevation would have resulted in loss of gingival architecture and the residual root of tooth No. 8. A confined subperiosteal tunnel and pouch were developed using the SMART instrumentation and surgical technique to allow the delivery of an anorganic bovine-derived bone mineral and platelet-derived growth factor combination and horizontally augment the labial alveolar bone in area Nos. 6, 7, and 8. The rationale for selecting the biomaterial was based on its low substitution rate and ability to maintain the contours of the augmentation (Figure 13 through Figure 15).55-57 The recombinant platelet-derived growth factor BB was used to stimulate bone formation and favor soft-tissue healing.58-60
The SMART method does not require the use of tenting screws or other space-maintaining devices. The degree of horizontal augmentation is a result of the ability to establish the confines of the tunnel and subperiosteal pouch, so that particle aggregation can be achieved while controlling the dispersion of the graft material.
Contrary to traditional GBR techniques, cell occlusive membranes are not required in the SMART method. The author previously conducted a pilot study that demonstrated membranes to be unnecessary for graft integration and mineralization. In addition, Simion and coworkers have reported that growth factor–mediated bone regeneration benefited when access to the periosteum was not prohibited by a barrier membrane.58 Because no decortication or intramarrow penetration was performed in the present case, the role of the periosteum as a potential source of osteoprogenitor cells in growth factor–mediated bone regeneration needs to be considered.61,62
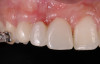
Because recession was already present on tooth No. 5, the scope of the SMART procedure was extended to horizontally augment adjacent areas that exhibited dehiscences and thin buccal plates (Figure 16). The augmented areas evident in Figure 16 (which can be compared to the preoperative imaging in Figure 4) were accomplished using two remote incisions. It must be emphasized, however, that this procedure may be technique sensitive, and predictable outcomes require training and experience. Unlike lateral subperiosteal techniques, the SMART method is based on the development of a laparoscopic tunnel from a remote incision to access the graft site. A subperiosteal pouch is subsequently created to confine the biomaterial particles (Figure 17 and Figure 18). This approach ensures that surgical trauma to the subperiosteum and the associated inflammatory reaction do not interfere with healing of the bone graft. Specially designed instruments are required to control the elevation of the periosteum, reach the graft site, and develop the subperiosteal pouch.
The bone graft underwent a 6-month maturation period to allow integration of the biomaterial. At this point, teeth Nos. 6 and 8 were atraumatically removed, and implants were immediately placed into the extraction sites without elevating a flap (Figure 19 through Figure 21). The gingival tissues exhibited a moderate degree of inflammation as a result of the difficult access for oral hygiene procedures under the post-orthodontic splint, which at the time had remained in place for 9 months. Implants featuring a tapered design were selected, and excellent primary stability was achieved (Figure 22 and Figure 23). Insertion torque values in excess of 45 Ncm were recorded for both implants, which allowed an immediate loading protocol.6,7,63,64
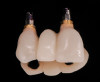
A screw-retained three-unit provisional prosthesis from teeth Nos. 6 through 8 was delivered during the same appointment (Figure 20). Additionally, direct composite occlusal overlays were bonded to the central fossae of the maxillary bicuspids and first molars to open the bite and disengage the maxillary anterior teeth, thus avoiding centric and excursive contacts during the osseointegration period.6-8,10,12
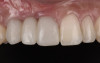
At the patient’s return visit 3 months after implant placement, the composite overlays were removed and the incisal edges of the provisional restoration were modified to approximate the length of the contralateral teeth. The patient reported no complaints, discomfort, or symptomatology throughout the osseointegration period. The implants were stable, and all discernable clinical parameters were within normal limits. The radiographic assessment revealed adequate bone-to-implant contact and osseous crest levels. Similarly, the peri-implant soft tissues displayed a healthy appearance and satisfactory gingival margin architecture (Figure 24 through Figure 27).
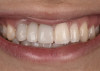
The patient subsequently enrolled in medical school, limiting her availability to continue treatment because of academic commitments and geographic location. As a result, she has been maintained with a long-term milled polymethylmethacrylate temporary restoration for an 18-month period and is currently scheduled to return for final impressions and completion of the definitive restoration.
Discussion
Predictability is essential when considering the treatment of high-risk scenarios in the esthetic zone. Therefore, the potential outcomes from bone-augmentation procedures must also be evaluated with regard to the achievement of adequate peri-implant soft-tissue architecture.
In this case report, the tridimensional reconstruction of a complex iatrogenic defect was accomplished using orthodontic forced eruption to restore the vertical height and minimally invasive bone grafting to achieve horizontal augmentation.27,29,48 This interdisciplinary approach allowed the predictable development and preservation of favorable peri-implant soft tissues. Gingival volume, margin level, and architecture were successfully restored while avoiding multiple hard- and soft-tissue grafting procedures. The only shortcoming was the length of the papillae adjacent to the area of the defect, which did not match the contralateral side.
The technique used provided adequate bone volume, while the soft-tissue disfigurements, complications, and morbidity associated with traditional GBR procedures were avoided.48 This procedure, however, may be technique sensitive, and predictable outcomes require the use of specially designed instruments, a specific surgical methodology, training, and experience.
Conclusion
Forced eruption in conjunction with minimally invasive bone grafting may provide synergistic advantages for the predictable and efficient reconstruction of gingival-alveolar defects in the esthetic zone. Further research and development are required to determine the full potential and limitations of this approach.
Acknowledgment
The author would like to thank LynAnn Mastaj, DMD, for her expert orthodontic advice and assistance with root torque mechanics.
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
About ther Author
Ernesto A. Lee, DMD
Clinical Professor and Director
Postdoctoral Periodontal Prosthesis Program
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
Private Practice
Bryn Mawr, Pennsylvania
References
1. Jensen AT, Jensen SS, Worsaae N. Complications related to bone augmentation procedures of localized defects in the alveolar ridge. A retrospective clinical study. Oral Maxillofac Surg. 2016;20(2):115-122.
2. Lei Q, Chen J, Jiang J, et al. Comparison of soft tissue healing around implants in beagle dogs: flap surgery versus flapless surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):e21-e27.
3. Chiapasco M, Romeo E, Casentini P, Rimondini L. Alveolar distraction osteogenesis vs. vertical guided bone regeneration for the correction of vertically deficient edentulous ridges: a 1-3-year prospective study on humans. Clin Oral Implants Res. 2004;15(1):82-95.
4. Jensen SS, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009;24(suppl):218-236.
5. Fürhauser R, Florescu D, Benesch T, et al. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin Oral Implants Res. 2005;16(6):639-644.
6. Wöhrle PS. Single-tooth replacement in the aesthetic zone with immediate provisionalization: fourteen consecutive case reports. Pract Periodontics Aesthet Dent. 1998;10(9):1107-1114.
7. Kan JY, Rungcharassaeng K. Immediate placement and provisionalization of maxillary anterior single implants: a surgical and prosthodontic rationale. Pract Periodontics Aesthet Dent. 2000;12(9):817-824.
8. De Rouck T, Collys K, Cosyn J. Immediate single-tooth implants in the anterior maxilla: a 1-year case cohort study on hard and soft tissue response. J Clin Periodontol. 2008;35(7):649-657.
9. Degidi M, Nardi D, Daprile G, Piatelli A. Buccal bone plate in the immediately placed and restored maxillary single implant: a 7-year retrospective study using computed tomography. Implant Dent. 2012;21(1):62-66.
10. Chu SJ, Salama MA, Salama H, et al. The dual-zone therapeutic concept of managing immediate implant placement and provisional restoration in anterior extraction sockets. Compend Contin Educ Dent. 2012;33(7):524-532.
11. Cooper LF, Reside GJ, Raes F, et al. Immediate provisionalization of dental implants placed in healed alveolar ridges and extraction sockets: a 5-year prospective evaluation. Int J Oral Maxillofac Implants. 2014;29(3):709-717.
12. Lee EA, Gonzalez-Martin O, Fiorellini J. Lingualized flapless implant placement into fresh extraction sockets preserves buccal alveolar bone: a cone beam computed tomography study. Int J Periodontics Restorative Dent. 2014;34(1):61-68.
13. Rieder D, Eggert J, Krafft T, et al. Impact of placement and restoration timing on single-implant esthetic outcome—a randomized clinical trial. Clin Oral Implants Res. 2016;27(2):e80-e86.
14. Chu SJ, Salama MA, Garber DA, et al. Flapless postextraction socket implant placement, part 2: the effects of bone grafting and provisional restoration on peri-implant soft tissue height and thickness—a retrospective study. Int J Periodontics Restorative Dent. 2015;35(6):803-809.
15. Morton D, Chen ST, Martin WC, et al. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. 2014;29(suppl):216-220.
16. Cosyn J, Eghbali A, Hermans A, et al. A 5-year prospective study on single immediate implants in the aesthetic zone. J Clin Periodontol. 2016;43(8):702-709.
17. Becker W, Dahlin C, Lekholm U, et al. Five-year evaluation of implants placed at extraction and with dehiscences and fenestration defects augmented with ePTFE membranes: results from a prospective multicenter study. Clin Implant Dent Relat Res.1999;1(1):27-32.
18. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(suppl):49-70.
19. Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009;20(suppl 4):113-123.
20. Santing HJ, Raghoebar GM, Vissink A, et al. Performance of the Straumann bone level implant system for anterior single-tooth replacements in augmented and nonaugmented sites: a prospective cohort study with 60 consecutive patients. Clin Oral Implants Res. 2013;24(8):941-948.
21. Cosyn J, Eghbali A, Hanselaer L, et al. Four modalities of single implant treatment in the anterior maxilla: a clinical, radiographic, and aesthetic evaluation. Clin Implant Dent Relat Res. 2013;15(4):517-530.
22. Cosyn J, Sabzevar MM, De Bruyn H. Predictors of inter-proximal and midfacial recession following single implant treatment in the anterior maxilla: a multivariate analysis. J Clin Periodontol. 2012;39(9):895-903.
23. Merli M, Migani M, Esposito M. Vertical ridge augmentation with autogenous bone grafts: resorbable barriers supported by ostheosynthesis plates versus titanium-reinforced barriers. A preliminary report of a blinded, randomized controlled clinical trial. Int J Oral Maxillofac Implants. 2007;22(3):373-382.
24. Merli M, Moscatelli M, Mariotti G, et al. Bone level variation after vertical ridge augmentation: resorbable barriers versus titanium-reinforced barriers. A 6-year double-blind randomized clinical trial. Int J Oral Maxillofac Implants. 2014;29(4):905-913.
25. Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. 1967;53(10):721-745.
26. Brown IS. The effect of orthodontic therapy on certain types of periodontal defects. I. Clinical findings. J Periodontol. 1973;44(12):742-756.
27. Ingber JS. Forced eruption. I. A method of treating isolated one and two wall infrabony osseous defects—rationale and case report. J Periodontol. 1974;45(4):199-206.
28. Pontoriero R, Celenza F Jr, Ricci G, Carnevale G. Rapid extrusion with fiber resection: a combined orthodontic-periodontic treatment modality. Int J Periodontics Restorative Dent. 1987;7(5):30-43.
29. Ingber JS. Forced eruption: alteration of soft tissue cosmetic deformities. Int J Periodontics Restorative Dent. 1989;9(6):416-425.
30. Salama H, Salama M. The role of orthodontic extrusive remodeling in the enhancement of soft and hard tissue profiles prior to implant placement: a systematic approach to the management of extraction site defects. Int J Periodontics Restorative Dent. 1993;13(4):312-333.
31. Van Venrooy JR, Vanarsdall RL. Tooth eruption: correlation of histologic and radiographic findings in the animal model with clinical and radiographic findings in humans. Int J Adult Orthodon Orthognath Surg. 1987;2(4):235-247.
32. Mantzikos T, Shamus I. Forced eruption and implant site development: soft tissue response. Am J Orthod Dentofacial Orthop. 1997;112 (6):596-606.
33. Amato F, Mirabella AD, Macca U, Tarnow DP. Implant site development by orthodontic forced extraction: a preliminary study. Int J Oral Maxillofac Implants. 2012;27(2):411-420.
34. Kent JN, Quinn JH, Zide MF, et al. Alveolar ridge augmentation using nonresorbable hydroxylapatite with or without autogenous cancellous bone. J Oral Maxillofac Surg. 1983;41(10):629-642.
35. Vanassche BJ, Stoelinga PJ, de Koomen HA, et al. Reconstruction of the severely resorbed mandible with interposed bone grafts and hydroxylapatite. A 2-3 year follow-up. Int J Oral Maxillofac Surg. 1988;17(3):157-160.
36. Block MS, Degen M. Horizontal ridge augmentation using human mineralized particulate bone: preliminary results. J Oral Maxillofac Surg. 2004;62(9 suppl 2):67-72.
37. Hasson O. Augmentation of deficient lateral alveolar ridge using the subperiosteal tunneling dissection approach. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):e14-e19.
38. Kfir E, Kfir V, Eliav E, Kaluski E. Minimally invasive guided bone regeneration. J Oral Implantol. 2007;33(4):205-210.
39. Dibart S, Sebaoun JD, Surmenian J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent. 2009;30(6):342-344,346,348-350.
40. Blanes RJ, Allen EP. The bilateral pedicle flap-tunnel technique: a new approach to cover connective tissue grafts. Int J Periodontics Restorative Dent. 1999;19(5):471-479.
41. Zabalegui I, Sicilia A, Cambra J, et al. Treatment of multiple adjacent gingival recessions with the tunnel subepithelial connective tissue graft: a clinical report. Int J Periodontics Restorative Dent. 1999;19(2):199-206.
42. Mahn DH. Treatment of gingival recession with a modified “tunnel” technique and an acellular dermal connective tissue allograft. Pract Proced Aesthet Dent. 2001;13(1):69-74.
43. Tözüm TF, Dini FM. Treatment of adjacent gingival recessions with subepithelial connective tissue grafts and the modified tunnel technique. Quintessence Int. 2003;34(1):7-13.
44. AlGhamdi AS, Buhite RJ. A new tunnel technique with acellular dermal matrix for soft tissue preparation prior to symphyseal block graft—a description of technique and case report. J Oral Implantol. 2008;34 (5):274-281.
45. Modaressi M, Wang HL. Tunneling procedure for root coverage using acellular dermal matrix: a case series. Int J Periodontics Restorative Dent. 2009;29(4):395-403.
46. Zadeh HH. Minimally invasive treatment of maxillary anterior gingival recession defects by vestibular incision subperiosteal tunnel access and platelet-derived growth factor BB. Int J Periodontics Restorative Dent. 2011;31(6):653-660.
47. Chao JC. A novel approach to root coverage: the pinhole surgical technique. Int J Periodontics Restorative Dent. 2012;32(5):521-531.
48. Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): a new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 2017;37(2):165-173.
49. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237-247.
50. Pieri F, Corinaldesi G, Fini M, et al. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2-year prospective study. J Periodontol. 2008;79(11):2093-2103.
51. Hämmerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19(1):19-25.
52. Urban IA, Nagursky H, Lozada JL, Nagy K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 25 patients. Int J Periodontics Restorative Dent. 2013;33(3):299–307.
53. Meloni SM, Jovanovic SA, Urban I, et al. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulated xenograft and autologous bone: a 1-year prospective clinical study. Clin Implant Dent Relat Res. 2017;19(1):38-45.
54. Urban IA, Monje A, Lozada JL, Wang HL. Long-term evaluation of peri-implant bone level after reconstruction of severely atrophic edentulous maxilla via vertical and horizontal guided bone regeneration in combination with sinus augmentation: a case series with 1 to 15 years of loading. Clin Implant Dent Relat Res. 2017;19(1):46-55.
55. Nevins ML, Camelo M, Nevins M, et al. Minimally invasive alveolar ridge augmentation procedure (tunneling technique) using rhPDGF-BB in combination with three matrices: a case series. Int J Periodontics Restorative Dent. 2009;29(4):371-383.
56. Buser D, Chappuis V, Bornstein MM, et al. Long-term stability of contour augmentation with early implant placement following single tooth extraction in the esthetic zone: a prospective, cross-sectional study in 41 patients with a 5- to 9-year follow-up. J Periodontol. 2013;84(11):1517-1527.
57. Jensen SS, Bosshardt DD, Gruber R, Buser D. Long-term stability of contour augmentation in the esthetic zone: histologic and histomorphometric evaluation of 12 human biopsies 14 to 80 months after augmentation. J Periodontol. 2014;85(11):1549-1556.
58. Simion M, Nevins M, Rocchietta I, et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009;29(3):245-255.
59. Cardaropoli D. Vertical ridge augmentation with the use of recombinant human platelet-derived growth factor-BB and bovine bone mineral: a case report. Int J Periodontics Restorative Dent. 2009;29(3):289-295.
60. Nevins M, Camelo M, Nevins ML, et al. Growth factor-mediated combination therapy to treat large local human alveolar ridge defects. Int J Periodontics Restorative Dent. 2012;32(3):263-271.
61. Zhu SJ, Choi BH, Huh JY, et al. A comparative qualitative histological analysis of tissue-engineered bone using bone marrow mesenchymal stem cells, alveolar bone cells, and periosteal cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(2):164-169.
62. Ceccarelli G, Graziano A, Benedetti L, et al. Osteogenic potential of human oral-periosteal cells (PCs) isolated from different oral origin: an in vitro study. J Cell Physiol. 2016;231(3):607-612.
63. Ottoni JM, Oliveira ZF, Mansini R, Cabral AM. Correlation between placement torque and survival of single-tooth implants. Int J Oral Maxillofac Implants. 2005;20(5):769-776.
64. Greenstein G, Cavallaro J. Implant insertion torque: its role in achieving primary stability of restorable dental implants. Compend Contin Educ Dent. 2017;38(2):88-95.