You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Globally, general dentists placed more than 5 million implants in 2017,1 with an expected average increase prevalence of 14% per year.2 The 2019 International Dental Show held in Cologne, Germany, had over 200 listed dental implant exhibitors, which speaks to the global market expansion and upsurge of multiple implant designs/components. While this is an exciting development for implant dentistry, such rapid growth can make it arduous for all clinicians to know implant maintenance best practice protocols. Planning, placing, and restoring an implant are all fundamental aspects for achieving positive outcomes, but the management of the implant quickly shifts to the dental hygienist for long-term success. This article is intended to elucidate the anatomic features of peri-implant tissues to support dental hygienists in assessing and planning for patient-centered care. Treatment of peri-implant disease and implant maintenance following treatment is also discussed.
Anatomic Features of Peri-implant Tissues
Although similar to periodontal health around natural teeth, the peri-implant anatomic features and functions do have significant structural differences, particularly concerning their involvement with surrounding tissues and biological attachment.3 From Greek origin, peri means "around" or "enclosing," and peri-implant is the area surrounding the implant/abutment that is composed of a soft-tissue zone, the peri-implant mucosa, which forms during the wound healing process,4 and the hard-tissue zone, which forms the attachment to the implant surface to achieve implant stability.4 The peri-implant mucosa facing the implant/abutment is often referred to as the biologic width and has been identified as having two separate sections: a coronal area, which is lined by a thin non-keratinized barrier epithelium and sulcular epithelium; and an apical area designated a zone of connective tissue adhesion, which appears to be in contact with the implant/abutment surface and varies between 1 mm and 2 mm thick.5 Although the mechanism of "attaching" or "leaning" of the epithelial attachment/adhesion to the implant/abutment surface is debatable,6 the key point is that peri-implant tissues are fragile and minimal.
Knowing the significant differences between periodontal and peri-implant tissues is pivotal in understanding the potential destructive patterns in the latter. For example, the numerous supportive, nutrition-supplying, and sensory dentoalveolar and gingival fiber group bundles associated with the connection of a natural tooth have only slight similarity to the fiber bundles found in peri-implant tissues.5 Therefore, when an instrument is selected for either assessing or debriding an implant/abutment, the peri-implant tissue biology and its dual function in protecting both the underlining bone and the bone supporting the implant must be taken into consideration. Any instrument used should limit invasiveness to the thin lining and delicate adhesion of the peri-implant mucosa.7
Etiology and Microbiology of Peri-implant Tissue Infections
Periodontal disease is multifactorial, and peri-implant tissue infections/diseases are susceptible to comparable risk factors. From a biological perspective, the primary etiology of peri-implant infection is the presence of a mature oral bacterial biofilm.4,5 The constant exposure to microorganisms in the oral microbiome challenges the peri-implant mucosa, including its biological adhesion (seal). Data from Tomasi et al suggests that the bacteria surrounding exposed dental implants forms a diverse microbiome regardless of the periodontal profile of the patient.8
The oral microbiome, like most biomes, is a complex ecosystem. Oral samples have identified more than 700 bacterial species, as well as other types of microbes.9 A patient's individual oral microbiome is typically in harmony and co-dependent (ie, commensal and a symbiotic microbiota).9 This is vital for healthy soft and hard dental tissues and human survival. When there is an imbalance (ie, dysbiotic microbial communities), it elicits an inflammatory host response that correlates to microbe-related pathologies such as peri-implant infections.9 A dysbiotic community can induce diseases through the regulation of keystone pathogens like Porphyromonas gingivalis, which can disrupt an entire commensal and symbiotic community. For example, all species, pathogenic or not, can be organized into a collective inflammophilic dysbiotic community,9 and this can be destructive to the peri-implant tissues.
Healthy biofilms are essential for oral and overall health. Clinicians typically are trained to treat any signs of biofilm using either hand or power instruments, and although this may be appropriate for addressing unfavorable conditions or preventing a situation from shifting to a dysbiotic state, professional judgment must be exercised in determining the ideal use of periodontal instrumentation. Such treatment must respect the peri-implant tissue biology and maintain harmony at the implant site. A risk-benefit judgment warrants consideration, especially in a healthy implant symbiotic site.
Mechanical disruption undoubtedly remains the best practice standard for treating peri-implant mucositis and maintaining peri-implant health,10 but what remains debatable is the ideal professional and patient self-care methodologies.
Dental Implant Instrumentation
Proper instrumentation, whether hand or powered, must adequately mechanically disrupt microbial deposits without altering the implant surface integrity, degrading the biomaterials, or damaging the peri-implant tissues. Currently, dental implant hygiene hand instruments are made of plastic (unfilled resin), graphite (filled resin), gold and titanium coated, or solid titanium. Many of these instruments may be sharpened, and some have replaceable working ends. These hand instruments may be purchased individually or in sets.
In studies using profilometric laser and scanning electron microscope (SEM) assessment of implant surface alterations, titanium, which is a highly biocompatible metal, resulted in less surface topography damage and trace elements as compared to plastic and graphite scalers.11,12 Several dental companies have produced titanium instruments with thinner working ends and increased tensile strength to effectively dislodge calculus or luting cements as compared to plastic instruments.
Power instrumentation inserts/tips (magnetostrictive or piezoelectric) are metal with a variance of cover sleeves and are essential in biofilm management. However, they are equally concerning as hand instrumentation with regard to affecting surface integrity, eliciting abutment alterations, and leaving plastic residues. Additionally, the lower vibration amplitude of movement may not produce a surface alteration but might indicate a lack of cleaning efficacy.12
Air polishing with low-abrasive powders such as glycine and erythritol has been proven to be safe and effective for supra- and subgingival biofilm management; additionally, it is comfortable for patients and is a time-saving methodology.13 Leung et al evaluated SLA (sand-blasted acid-etched) titanium discs inoculated with dental plaque and anaerobically incubated for 21 days, allowing biofilm to mature and completely obscure the disc surfaces. Glycine powder delivered with a prophylaxis device resulted in a statistically significant decontaminated implant surface.14 These results align with those published by Cobb et al, which identified biofilm effectiveness and safety in relation to implant surfaces and biomaterials (ie, abutment and prosthetics) when using glycine powder versus sodium bicarbonate powders.15
Petersilka et al used light microscopy to evaluate damaged gingival epithelium. They recorded histological scores to quantify gingival damage immediately after hand instrumentation and at glycine application at 1 and 14 days later. The glycine group resulted in minor erosion scores, whereas positive control (hand) specimens displayed moderate to severe erosion scores. At 14 days after hand instrumentation, all groups returned to intact baseline.16
Currently, there are numerous implant surfaces (eg, rough, smooth, titanium, zirconia) and various biomaterials for abutments and prostheses (eg, zirconia, gold, porcelain). The exposure of implant threads or abutment surfaces at the time of therapy can render mechanical disruption or hard deposit removal (luting cement or calculus) difficult. Given the plethora of options available in providing therapy, dental hygienists must recognize that all instrumentation used on implant/abutment surfaces will have some level of effect on the tissues – the only question is to what extent.
To support critical decision-making in selecting a methodology, a number of factors must be considered. The chosen instrumentation methodology must take into account the peri-implant tissue health and any etiology and its contributing factors. Additionally, the methodology should support the disruption of biofilm or removal of hard deposits while respecting the biology of the peri-implant tissues. Ideally, it should be minimally invasive.
Risk Indicators
For periodontally involved patients, all risk indicators need to be determined/assessed. Periodontal diseases have no cure, but rather the risk indicators/factors must be managed. Given their pathologic nature, these diseases may help identify what needs to be "corrected" to improve the imbalance that exists when disease is present. Indicators noted by Heitz-Mayfield and Salvi for peri-implant infections such as peri-implant mucositis and peri-implantitis include biofilm accumulation, smoking, and radiation.10
Ongoing peri-implant therapy is crucial to the maintenance of peri-implant health, particularly treatment of peri-implant mucositis, with the goal of limiting the potential progression to peri-implantitis.10 With regard to compliance, patients not adhering to hygiene intervals (3 to 6 months, depending on disease) had a prevalence of 48% for developing peri-implant mucositis during a 9- to 14-year observation period.10 Several systematic reviews have identified a history of periodontitis as a significant risk factor for increased frequency of biological complications around dental implants.11 Moreover, individuals with a history of aggressive periodontitis have been found to be at greater risk for peri-implant disease and implant loss.12 Cement-retained restorations also may increase the risk for peri-implant diseases, because residual cement may act as a plaque-retentive factor. Cement remnants were frequently found around dental implant-supported cement-retained prostheses, regardless of the efforts made in removing them following delivery of the restorations.13
Peri-implant soft-tissue deficiencies, such as lack of tissue volume and keratinization, may also play a role in the incidence of peri-implant diseases. It has been demonstrated that dental implants with inadequate peri-implant soft tissues have worse peri-implant parameters (increased plaque index, gingival index, and bleeding on probing) and are at greater risk of peri-implant bone loss and mucosal recession than implants with adequate peri-implant soft tissues.14
Treatment of Peri-implant Diseases
With increasing dental implant use, the prevalence of peri-implant complications can also be expected to rise. Inflammatory reactions of peri-implant tissues may occur due to several reasons, including prolonged bacterial biofilm exposure, failure of hardware components (eg, implant fractures, improper fit of the implant-abutment interface, etc), and oral pathological entities such as peripheral giant cell granulomas. The most current descriptions of peri-implant diseases come from the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions (Table 1).3 If peri-implant disease develops during the maintenance phase, appropriate action should be taken (Table 2).17
Peri-implant Mucositis
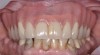
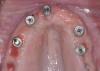
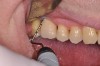
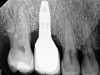
The main objective of peri-implant mucositis treatment is the resolution of inflammation.18 Oral healthcare providers should employ a two-pronged approach in the treatment of peri-implant mucositis. First, they should consider the patient's ability to perform adequate oral hygiene around the implant prosthesis—this includes assessing the patient's accessibility to the area in the mouth—and prescribe appropriate aids in biofilm removal (Figure 1 and Figure 2). Second, the clinician should institute mechanical therapy and an appropriate peri-implant maintenance regimen consisting of further evaluation and treatment, if needed.
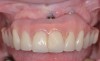
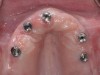
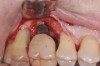
The clinician should review and reinforce with the patient the patient-administered oral hygiene efforts. Use of an intraoral stain to demonstrate inadequate plaque control has been shown to be effective in patient motivation.19 Nonsurgical peri-implant treatment should consist of mechanical removal of the bacterial biofilms and elimination or control of all local plaque retentive factors. Curettes, sonic and ultrasonic instruments, as well as air polishing with glycine powder are all effective methods of biofilm removal.20 The use of local antibacterials and local or systemic antibiotics appear to have minimal benefit.21 Once the treatment of peri-implant mucositis has been rendered, it is very important that the oral healthcare provider evaluate the response to therapy (Figure 3 and Figure 4). If initial therapy fails to resolve the peri-implant inflammation, it may be beneficial to assess the marginal fit and design of the implant prosthesis with regard to patient access, as well as the possible presence of cement remnants.
Peri-implantitis
Following the diagnosis of peri-implantitis (Figure 5 and Figure 6), an initial evaluation of the affected implant fixture should be made to establish the rationale for treatment and whether removal of the implant is indicated. Although nonsurgical treatment of peri-implantitis may not always be successful, it should always precede surgical therapy, as this will provide the clinician time to assess the patient's oral hygiene status and response to therapy.22 The primary goal of surgical treatment of peri-implantitis is the decontamination of the denuded implant surface. Multiple surface decontamination protocols have been described in the literature; however, to date, none have shown superiority.23
After implant surface decontamination, the specific surgical approach (eg, access surgery, respective, or regenerative) for each case is heavily dependent on the position of the implant in the oral cavity, as well as the configuration of the peri-implant defect (Figure 7).24 In a recent meta-analysis, an average 2 mm to 3 mm peri-implant probing depth reduction was achieved following surgical intervention.25 Also, implant surface modification (implantoplasty) (Figure 8) was superior to access flap alone. The addition of bone grafts yielded an average of 2 mm of bone fill in defects treated with a regenerative approach. Regenerative treatments may be contraindicated in smokers.
Peri-implant Soft-tissue Deficiencies
Peri-implant soft-tissue dimensions should also be assessed during dental implant maintenance. Peri-implant soft-tissue deficiencies, such as lack of keratinization and lack of volume, may be contributing factors to the occurrence of peri-implant diseases. Peri-implant tissue thickness of 2 mm and >2 mm of keratinized mucosa are considered adequate to maintain peri-implant tissue health and reduce the incidence of peri-implant complications.26 Augmentation of soft-tissue-deficient implant sites showed a consistent improvement in peri-implant soft-tissue parameters and reduced probing depths.27 Furthermore, peri-implant soft-tissue augmentation may reduce the risk of peri-implant diseases in patients with erratic compliance to their dental implant maintenance.28
Dental Implant Maintenance Following Treatment of Peri-implant Diseases
After peri-implant disease has been treated, it is imperative that a customized peri-implant maintenance program be drawn up and followed.29 This maintenance program should take all risk factors into account, and an individualized maintenance plan should be tailored to each patient. Implants that have previously undergone surgical or nonsurgical treatment should be appropriately assessed to confirm peri-implant tissue health at every maintenance appointment. A recent systematic review reported that regular peri-implant maintenance around dental implants treated for peri-implantitis resulted in stable clinical parameters and bone levels in most of the patients.30 Initial bone loss and defect configuration (circumferential versus one or two sites) are the main risk factors during maintenance that may affect implant success and survival rates.31
Lack of implant maintenance following treatment of peri-implant diseases will eventually result in further peri-implant bone loss and a decrease in dental implant survival.29 The incidence of peri-implant diseases during maintenance is almost 6 times higher for noncompliant patients, and bacterial loads are greater.32 Patients with erratic and no compliance were more frequently diagnosed with peri-implantitis (30% versus 2.4%) compared to patients with regular compliance (two or more maintenance visits per year). A previous history of periodontitis, disease severity, and smoking habits were factors that would reduce the compliance rate of dental implant maintenance.33
Conclusion
To correctly identify implant-related diseases and implement effective, safe, and minimally invasive therapeutic modalities that could significantly impact implant success and survival, oral healthcare providers, including dental hygienists, should be well-versed in the fundamental principles of implant and abutment designs, as well as the features and functions of peri-implant tissues. Maintenance protocols, including professional biofilm management and oral self-care routines, must be comprehensive in assessing, diagnosing, and continuously evaluating the peri-implant tissues, both soft and hard, for patient-centered long-term success.
About the Authors
Penny Hatzimanolakis, Dipl. DH, BDSc, MSc
Clinical Associate Professor, Department of Oral Biological and Medical Sciences, University of British Columbia, Vancouver, British Columbia, Canada
Ioannis Tsourounakis, DDS, MSc, Cert. Perio
Part-time Clinical Instructor, Dr. Gerald Niznick College of Dentistry, University of Manitoba, Winnipeg, Manitoba, Canada; Private Practice, Winnipeg, Manitoba, Canada
Anastasia Kelekis-Cholakis, DMD, Dipl. Perio
Division Head of Periodontics and Director of Graduate Periodontal Program, Dr. Gerald Niznick College of Dentistry, University of Manitoba, Winnipeg, Manitoba, Canada
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Elani HW, Starr JR, DaSilva JD, Gallucci GO. Trends in dental implant use in the U.S., 1999-2016, and projections to 2026. J Dent Res. 2018;97
(13):1424-1430.
2. Gaviria L, Salcido JP, Guda T, Ong JL. Current trends in dental implants. J Korean Assoc Oral Maxillofac Surg. 2014;40(2):50-60.
3. Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45 suppl 20:S278-S285.
4. Berglundh T, Abrahamsson I, Welander M, et al. Morphogenesis of the peri-implant mucosa: an experimental study in dogs. Clin Oral Implants Res. 2007;18(1):1-8.
5. Araujo MG, Lindhe J. Peri-implant health. J Periodontol. 2018;89 suppl 1:S249-S256.
6. Larjava H, Koivisto L, Hakkinen L, Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res. 2011;90(12):1367-1376.
7. Soadoun AP, Touati B. Soft tissue recession around implants: Is it still unavoidable? Part II. Pract Proced Aesthet Dent. 2007;19(2):81-87.
8. Tomasi C, Tessarolo F, Caola I, et al. Early healing of peri-implant mucosa in man. J Clin Periodontol. 2016;43(10):816-824.
9. Cortés-Acha B, Figueiredo R, Seminago R, et al. Microbiota analysis of biofilms on experimental abutments mimicking dental implants: an in vivo model. J Periodontol. 2017;88(10):1090-1104.
10. Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Clin Periodontol. 2018;89 suppl 1:S257-S266.
11. Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23(6):643-658.
12. de Almeida Curylofo F, Araújo Barbosa L, Roselino AL, et al. Instrumentation of dental implants: a literature review. RSBO. 2013;10(1):82-88.
13. Lu H, He L, Zhao Y, Meng H. The effect of supragingival glycine air polishing on periodontitis during maintenance therapy: a randomized controlled trial. PeerJ. 2018;6:e4371. doi: 10.7717/peerj.4371.
14. Karr Yee Leung KE. Decontamination of rough implant surfaces in vitro using glycine powder air polishing [thesis]. Vancouver, Canada; The University of British Columbia; 2018. https://open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0370947. Accessed May 31, 2019.
15. Cobb CM, Daubert DM, Davis K, et al. Consensus conference findings on supragingival and subgingival air polishing. Compend Contin Educ Dent. 2017;38(2):e1-e4.
16. Petersilka G, Faggion CM Jr, Stratmann U, et al. Effect of glycine powder air-polishing on the gingiva. J Clin Periodontol. 2008;35(4):324-332.
17. Todescan S, Lavigne S, Kelekis-Cholakis A. Guidance for the mainten-ance care of dental implants: clinical review. J Can Dent Assoc. 2012;78:c107.
18. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 suppl 20:S286-S291.
19. Renvert S, Giovannoli JL. Peri-implantitis. Paris, France: Quintessence Publishing; 2012.
20. Kelekis-Cholakis A, Rothney J. Maintenance of implant patients: a narrative review. Implant Dent. 2019;28(2):161-172.
21. Kelekis-Cholakis A, Atout R, Hamdan N, Tsourounakis I. Peri-Implant Complications: A Clinical Guide to Diagnosis and Treatment. Cham, Switzerland: Springer; 2018.
22. Renvert S, Quirynen M. Risk indicators for peri-implantitis. A narrative review. Clin Oral Implants Res. 2015;26 suppl 11:15-44.
23. Froum SJ, Dagba AS, Shi Y, et al. Successful surgical protocols in the treatment of peri-implantitis: a narrative review of the literature. Implant Dent. 2016;25(3):416-426.
24. Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010;37(5):449-455.
25. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041.
26. Jung RE, Heitz-Mayfield L, Schwarz F, Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 3-Aesthetics of peri-implant soft tissues. Clin Oral Implants Res. 2018;29 suppl 15:14-17.
27. Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29 suppl 15:32-49.
28. Monje A, Blasi G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J Periodontol. 2019;90(5):445-453.
29. Ramanauskaite A, Tervonen T. The efficacy of supportive peri-implant therapies in preventing peri-implantitis and implant loss: a systematic review of the literature. J Oral Maxillofac Res. 2016;7(3):e12.
30. Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res. 2018;29 suppl 16:331-350.
31. Serino G, Turri A, Lang NP. Maintenance therapy in patients following the surgical treatment of peri-implantitis: a 5-year follow-up study. Clin Oral Implants Res. 2015;26(8):950-956.
32. Costa FO, Takenaka-Martinez S, Cota LO, et al. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39(2):173-181.
33. Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017;88(10):1030-1041.