You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The development of practical adhesive dentistry can be traced to Dr. Michael Buonocore who, in 1955, discovered he could increase the retention of acrylic-based restoratives by first treating the enamel with phosphoric acid.1 Subsequent research by Buonocore, Gwinnett, and Matsui elucidated the mechanism of adhesion as micromechanical attachment via resin infiltration and tag formation within the acid-demineralized enamel.2 While long-term bonding to phosphoric-acid–etched enamel surfaces has proven to be highly reliable and predictable, long-term bonding to dentin has been considerably more problematic. This is largely due to morphologic, histologic, and compositional differences between the two substrates.3 For one thing, dentin is a vital, dynamic, and highly variable substrate. Superficial, middle, and deep dentin can vary significantly in structural, physical, and chemical composition. Enamel, conversely, is quite consistent throughout and is also considerably more mineralized than dentin. The inorganic content of mature enamel is approximately 96% hydroxyapatite by weight; the remainder consists of water and organic material. Dentin, on the other hand, is approximately 70% hydroxyapatite by weight, 18% organic material (predominantly type I collagen), and 12% water.4,5 These percentages are not consistent and can vary significantly depending on several factors, including dentin depth, age of the teeth, and history of tooth trauma and/or pathology. This, coupled with the relatively high water content of dentin, presents a significant challenge for consistent and reliable long-term adhesion.
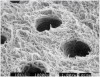
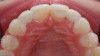
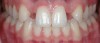
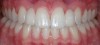
The few available adhesive systems of the 1970s and early 1980s were relatively hydrophobic in nature and unable to adequately penetrate the dentin smear layer, thus limiting their direct interaction with the tooth tissues. The smear layer is the residue that is left on the surface of the dentin after rotary instrumentation with diamond or carbide burs (Figure 1). It is a thin amorphous layer largely composed of degraded collagen, bacteria, and various inorganic dentin and enamel debris.6,7 Early adhesive systems were extremely limited and generally ineffective, in part because they bonded directly to the smear layer and were, thus, limited by the smear layer’s low intrinsic cohesive strength.8 Eventually, it was recognized that the smear layer needed to be removed and/or modified and bypassed in some fashion so that adhesive primers and resins could interact directly with the dentin. In the case of total-etch adhesive systems, the smear layer is essentially dissolved with phosphoric acid (H3PO4) and subsequently washed away during the rinsing step (Figure 2 and Figure 3). With self-etching systems, various acidic primers are used to modify, disrupt, and/or solubilize the smear layer and, although the remnants are not washed away as with total-etch systems, still permit direct adhesive interaction with the dentin substrate.9
The acids and/or acidic primers and conditioners used with either total- or self-etching bonding systems do not just remove and/or disrupt the smear layer but create a thin zone of demineralization, exposing collagen fibrils that are either subsequently (total-etch) or concurrently (self-etch) infiltrated with various functional and cross-linking primers and resins. One of the goals in developing a successful adhesive interface is the infiltration and penetration through this acid-demineralized zone with various primers and/or resins that can be subsequently polymerized by light and/or chemical curing mechanisms. It is this thin layer of resin-infiltrated dentin, first described in a classic 1982 paper by Nakabayashi and colleagues,10 that is called the hybrid layer (Figure 4). Although micromechanical resin infiltration and entanglement with the tooth tissues appears to be the primary attachment mechanism to both enamel and dentin, strong evidence suggests that certain monomers (such as 10-MDP) chemically interact, via ionic bonding, to calcium in hydroxyapatite as well.11,12 The hybrid layer and associated resin tags form a thin polymerized micromechanically, and in some cases chemically, attached resinous surface layer that acts as the foundation for subsequently placed chemically compatible restorative materials and resin-based cements.
While an in-depth discussion of the imperfect, and sometimes confusing, classification categories typically used to describe adhesive systems (etch-and-rinse, etch-and-no rinse, three-bottle, two-bottle, one-bottle, all-in-one, various generations, etc.) is beyond the scope of this article, it is reasonably safe to say that all adhesive systems, regardless of classification category, contain some type of acidic conditioner, dentin primer, and bonding resin. For example, the basic protocol when using a three-step total-etch system (4th generation) is the sequential placement of the three primary components (acidic conditioner, hydrophilic primer typically consisting of HEMA and adhesive functional monomer(s), and hydrophobic resin). In the case of 4th generation systems the components are packaged in separate containers and applied sequentially. Typically, phosphoric acid is first placed on enamel and dentin for a period of time, rinsed off with water, then a hydrophilic primer is placed and air-dried, followed by the placement of a separate, relatively hydrophobic bonding resin. The simplified two- and one-bottle systems still contain the aforementioned three primary components, but the components are consolidated and combined in various configurations depending on the specific system. In any case, as most dentists know, the trend in recent years has been toward not just the simplification of adhesive systems, but making them universal as well.
Table 1 lists eight products that are marketed as universal adhesives, along with their basic chemistries.
What is a “Universal” Adhesive?
There does not appear to be any “official” definition as to what qualifies as a universal adhesive. In any case, universal adhesives should not be confused with 7th generation self-etching single-bottle or “all-in-one” systems such as iBond® (Heraeus Kulzer), Xeno® IV (DENTSPLY Caulk), Clearfil™ S3 Bond (Kuraray), and OptiBond® All-In-One (Kerr Corporation). For one thing, universal adhesives are said to have much broader applications than 7th generation systems. Universal adhesives have been described by some manufacturers and opinion leaders as: ideally a single-bottle, no-mix, adhesive system that can be used in total-etch, self-etch, or selective-etch mode depending on the specific clinical situation and personal preferences of the operator.13,14 Additionally, manufacturers typically state that universal adhesives can be used for the placement of both direct and indirect restorations and are compatible with self-cure, light-cure, and dual-cure resin-based cements. It is further stated that universal adhesives can be used not only to bond to dentin and enamel, but as adhesive primers on substrates such as zirconia, noble and non-precious metals, composites, and various silica-based ceramics. In principle, this would enable bonding to these surfaces without the need for dedicated and separately placed primers such as silane and various products marketed as metal and zirconia primers.
If this unofficial definition as to what constitutes a universal adhesive is accepted, it becomes apparent that a degree of ambiguity exists as to where certain products that are sometimes marketed as universal adhesives actually fit in. For example, OptiBond™ XTR (Kerr Corp.) is a two-bottle system that the manufacturer describes on its website as a “self-etch, light-cure, universal dental adhesive” that can be used for direct and indirect restorations, is compatible with light-, self-, and dual-cure resin cements, and bonds to metals, zirconia, porcelain, and composite. While this may all be true, as a two-bottle self-etching system with individual components that are applied separately and in a sequential fashion when bonding to tooth tissues, would OptiBond XTR be better described as a 6th generation self-etching system with expanded functions? Some products marketed as universal adhesives, such as Futurabond Universal Bond (VOCO), require the mixing of two separate components prior to use. This product comes in individual disposable blister packs that are easily activated just prior to use. There may be very sound reasons for keeping the chemistry of an adhesive separate until just prior to use in terms of stability and product performance, but where does this mixing requirement fit into the definition of a universal adhesive? Prelude One™ (Danville Materials) is marketed on the company’s website as a “self-etch bonding system” yet the manufacturer claims it’s not contradictory to also use it in total-etch capacity (S. Chen, Danville Materials, personal conversation/e-mail). Scotchbond™ Universal (3M ESPE) and Clearfil™ Universal (Kuraray) require a separate “activator” be added to the adhesive if another manufacturer’s self- or dual-cure resin cement is used during placement of indirect restorations, while Prime&Bond Elect® (DENTSPLY Caulk) requires the addition of a separate activator for all self- and dual-cure resin cements. In addition, the manufacturers of some universal adhesives still recommend the use of separate and dedicated primers to optimize bond strength to substrates such as porcelain and zirconia.
Thus, it appears, at least in certain situations and with some products, that universal adhesives actually consist of two bottles, or require the use of an additional activator, or have chemistries that must be mixed prior to use, or bond most optimally to porcelain and zirconia with separately applied and dedicated primers, or are not compatible with a total-etch protocol. Suddenly, the definition of what qualifies as a universal adhesive becomes a bit muddled. Does this mean some products should not be classified as universal adhesives, or does the definition of exactly what a universal adhesive is need to be broadened? In any case, can universal adhesives, however they are defined, really do all of the things they are said to be able to do? Clearly, there must be significant practical and chemical challenges in developing such a versatile product, placing all the chemistry required into one or even two bottles, have it perform as claimed, and have it remain stable for a reasonable period of time. So, how is it done?
It’s All About the Chemistry
In order to develop a truly universal adhesive, very specific and synergistic functional and cross-linking monomers that are multifunctional in nature are required. They must be capable of reacting with a number of different substrates, be able to copolymerize with chemically compatible resin-based restoratives and cements, and have some hydrophilic character in order to properly “wet” dentin that has a significant water content, yet at the same time be as hydrophobic as possible once polymerized to discourage hydrolysis and water sorption over time. Film thickness of the polymerized adhesive must also be thin enough as to not interfere with the seating of indirect restorations. In addition, universal adhesives ideally should be acidic enough to be effective in a self-etching mode but not so acidic that they breakdown initiators needed for the polymerization of self- and dual-cure resin cements.15
Universal adhesives must also contain water, as it is required for dissociation of the acidic functional monomers, inherent in all these systems, that makes self-etching possible. One of many dilemmas faced by chemists developing universal adhesives is that while some water is needed, too much can degrade the chemistry of these systems, contribute to phase separation of monomers, decrease shelf-life, and be difficult to completely evaporate during the air-drying step.16,17 Residual water after air-drying could result in incomplete adhesive polymerization, increased hydrolysis after polymerization, and a generally compromised adhesive interface. Adding ethanol or acetone into universal adhesive formulations enhances resin wetting and infiltration of tooth tissues and also aids in water removal and evaporation during the air-drying step. There are many other important subtle factors and nuances that vary from manufacturer to manufacturer, such as pH, initiator and solvent chemistry, and specific monomer types and ratios, that also play a vital role in the viability of these systems and, in some situations, give certain products advantages over others. An examination of the chemistry of the systems listed in Table 1 shows some remarkable similarities, as well as some subtle and important differences.
The 10-MDP Adhesive Functional Monomer
All of the universal adhesives listed in Table 1 use phosphate esters (R-O-PO3H2) as their primary adhesive functional monomer. In fact, phosphate esters form the backbone of virtually all current universal adhesive systems and enable them to do much of what they do. These monomers have many positive attributes, including the potential to bond chemically to metals,18 zirconia,19 and to tooth tissues through the formation of non-soluble Ca++ salts.11,12 In addition, their acidic nature (they are esters of phosphoric acid) gives them the potential to etch and demineralize tooth tissues, which makes them good candidates for use in adhesives that require self-, selective-, and total-etching options.
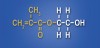
A very practical and proven phosphate ester, which also happens to be the one used in the formulations of many current universal adhesives, was actually synthesized more than 30 years ago. In the early 1980s chemists at Kuraray (Osaka, Japan) synthesized the adhesive functional monomer 10-MDP (methacryloyloxy-decyl-dihydrogen-phosphate) (Figure 5). One of the first practical applications of this new monomer was its use in the company’s Panavia™ adhesive resin cement. Panavia proved to be especially adept at bonding to metals, and its success led to the use of the MDP monomer in virtually all subsequent Kuraray adhesives. The 10-MDP monomer has many positive attributes that make it practical for use in a universal adhesive. It is a versatile amphiphilic functional monomer with a hydrophobic methacrylate group on one end (capable of chemical bonding to methacrylate-based restoratives and cements) and a hydrophilic polar phosphate group on the other (capable of chemical bonding to tooth tissues, metals, and zirconia). This attribute alone makes it desirable for use in a “universal” adhesive. Moreover, the long carbon chain backbone of the monomer renders it fairly hydrophobic. In fact, with a partition coefficient of 4.1 (partition coefficient is essentially a measure of how hydrophilic or hydrophobic a chemical substance is) 10-MDP is the most hydrophobic of all the functional monomers typically used in dental adhesives.20 This may be important in terms of durability, as water sorption and hydrolytic breakdown of the adhesive interface over time has been implicated as one of the primary causes of bond failure.21,22 It makes sense that, once they are placed and polymerized, adhesive monomers that discourage water sorption are desirable. The hydrophobic nature of 10-MDP also makes it relatively stable in solution, which is important in terms of shelf-life. Additionally, 10-MDP is one of the few monomers used in adhesive dentistry that has been shown to actually bond chemically to the tooth tissues via ionic bonding to calcium found in hydroxyapatite (Ca10[PO4]6[OH]2).11,12 Stable MDP-calcium salts are formed during this reaction and deposited in self-assembled nano-layers of varying degrees and quality depending on the adhesive system.23,24 This type of molecular interaction and self-organization, coupled with the relatively hydrophobic nature of polymerized 10-MDP, helps explain why this monomer appears to be so effective in creating adhesive interfaces that are resistant to biodegradation23 (J. Oxman, chemist, 3M ESPE, personal communication).
When Kuraray’s patent on 10-MDP expired (around 2003) other manufacturers began to explore its potential. In October 2009, Bisco, Inc. introduced a dedicated zirconia primer, Z-Prime™, that utilized a combination of 10-MDP and biphenyl dimethacrylate (BPDM) in its formulation (C. Suh, Bisco, Inc., personal communication). The use of secondary ion mass spectrometry (SIMS) at Northwestern University showed evidence of actual chemical bonding between the 10-MDP in Z-Prime and zirconia19 and the product received very favorable reviews for use as a dedicated zirconia primer25 (Figure 6 through Figure 11). Two years after the introduction of Z-Prime, 3M ESPE introduced the first “universal” adhesive (Scotchbond Universal), which also utilized 10-MDP in its formulation (J. Fundingsland, 3M ESPE, personal communication). This product was followed shortly thereafter by Bisco’s All-Bond Universal®, also featuring the 10-MDP monomer. In fact, six of the eight universal adhesives listed in Table 1 use 10-MDP in their formulations (including one that uses a modified 10-MDP [information provided by G. Connell, Director of Clinical Education & North American Training, VOCO Canada]). In addition to 10-MDP, other phosphate esters such as PENTA-P (dipentaerythritol penta acrylate monophosphate) and GPDM (glycero-phosphate dimethacrylate) also appear to be viable alternatives. In any case, it is apparent that the use of phosphate esters is an important part of the universal adhesive story, but it’s only part of the story.
The Delicate Balance Between Hydrophilic, Hydrophobic, And Adhesive Functional Monomers
A great paradox of adhesive dentistry is that the chemistry that helps make adhesive systems so effective initially can also contribute to their eventual breakdown. One reason for the success of current adhesive systems is the use of hydrophilic monomers that are able to interact with and “wet” tooth tissues that are, to some degree, inherently moist. The problem is that the same hydrophilic groups that initially facilitate primer/resin interaction with the tooth tissues can become a liability in the long term by encouraging water sorption and hydrolysis of the adhesive interface.26 Indeed, one of the major problems with 7th generation single-bottle self-etching systems is that the inherently hydrophilic nature of the polymerized adhesive, coupled with residual water that may remain, has been shown to act as a semi-permeable membrane permitting water diffusion that, over time, could lead to hydrolysis and breakdown of the adhesive interface.27 One could argue that an “ideal” dentin bonding agent would be one that is hydrophilic when first placed (to facilitate interaction with the tooth tissues) but then becomes hydrophobic once polymerized (to discourage water sorption). The next best thing would be gradation from hydrophilic to hydrophobic as one moves from the tooth surface to the restorative interface.28 Indeed, this is the strategy employed by the original three-step total-etch (4th generation) and two-step self-etch (6th generation) adhesive systems—that is, the initial placement of a hydrophilic primer, which is then overlaid by a relatively hydrophobic bonding resin.
Chemists developing single-bottle universal adhesives had to formulate an optimized blend of chemically compatible hydrophobic, adhesive functional, and hydrophilic monomers that would work in a synergistic fashion and when polymerized form a durable, and hopefully hydrophobic, bonded interface. In this regard, the different monomers employed in universal adhesives each have their own specific functions. Generally, the hydrophilic ends of monomers interact with the tooth tissues, while the hydrophobic ends interact with methacrylate-based restorative materials or cross-link with other functional and structural monomers. The terminal ends of some adhesive functional monomers are hydrophilic initially (such as the ionic phosphate group in 10-MDP) but become more hydrophobic once they chemically react with tooth tissues and are polymerized.
There are significant challenges in striking just the right balance between hydrophilic and hydrophobic character when developing a universal adhesive, as the monomers need to initially be hydrophilic enough to wet, infiltrate, and interact with the dentin substrate, but once they are polymerized, not so hydrophilic that they encourage water sorption that could lead to hydrolysis and breakdown of the adhesive interface over time. Manufacturers of universal adhesives address these issues by blending some well known and widely used monomers such as bis-GMA (hydrophobic) and hydroxyethyl methacrylate (HEMA) (hydrophilic) along with proprietary and various adhesive functional monomers that vary from manufacturer to manufacturer. One of the goals after placing, drying, and curing a universal adhesive should be the creation of a highly cross-linked hydrophobic polymer matrix29 that is well bonded to the tooth tissues on one end and to restorative materials, such as composites, on the other. Some manufacturers may have advantages over others in this regard.
Almost all adhesive systems, including universal systems, utilize HEMA in their formulations (Figure 12). HEMA is a versatile low molecular weight hydrophilic monomer that is particularly adept at infiltrating and “wetting” dentin substrates. It is extremely soluble in water, ethanol, and acetone, and thus easy to incorporate into adhesive formulations. The hydrophilicity of HEMA makes it an excellent adhesion-promoting monomer that has been shown to improve immediate bond strengths of adhesive systems by enhancing monomer diffusion into dentin and facilitating the formation of the “hybrid” layer.30-32 HEMA is frequently added to adhesives, not only to ensure good wetting, but also because of its solvent-like nature. This improves stability and helps keep hydrophobic and hydrophilic monomers in solution by minimizing phase separation in the presence of water (HEMA-free adhesives can have issues with phase separation).30,33,34
While HEMA has many positive attributes there is also a downside. HEMA in both the uncured and polymerized state readily absorbs water. Once polymerized it can swell, discolor, and contribute to hydrolysis of the adhesive interface.29,32,35 High amounts of HEMA can also decrease mechanical properties of the resulting polymer. Uncured HEMA also has the potential to lower the vapor pressure of water and may make it more difficult to evaporate during the air-drying step.36 While the concentration of HEMA used in universal adhesives varies from manufacturer to manufacturer, the goal should be to optimize the HEMA concentration (use least amount possible) to take advantage of the benefits of this monomer while concurrently minimizing its undesirable properties.
The pH Puzzle
The pH of current universal adhesives varies from about 2.2 to 3.2 depending on the product. Universal adhesives are generally considered to have “mild” (pH > 2) or “extra-mild” (pH > 2.5) etching capabilities.37,38 Adhesives in this pH range can be very effective in terms of bonding to dentin. The concern is that they may not be as effective when it comes to bonding to enamel (especially to uncut enamel).39-41 As an example, a popular two-bottle 6th generation self-etching system (Clearfil™ SE Bond, Kuraray) with a pH in the range of current universal adhesives and which also contains the 10-MDP monomer has been shown to bond more predictably to enamel in some clinical studies when the enamel is first etched with phosphoric acid.42-44 In fact, a common clinical technique when using this product is to first etch the enamel with traditional phosphoric acid gel (selective-etch technique). While there are in-vitro studies that do demonstrate acceptable bond strength values to enamel using the self-etching mode of some universal adhesives,45,46 caution is urged as there is equivocation in the literature47-50 and some systems may perform significantly better (or worse) than others when it comes to enamel bonding.51 The author’s personal preference, and recommendation, is to use universal adhesives only in the selective- or total-etching modes when enamel is present to ensure predictable long-term bonding to this substrate. In the case of bonding in full-coverage restorations, where there is little or no enamel remaining, then universal adhesives used in the self-etching mode is a viable, and perhaps even preferred, option.
There is also a direct correlation between pH and the compatibility of universal adhesives with self- and dual-cure resin cements and composites. As a generalization, the more acidic the adhesive the less compatible it is with the self-cure mode of dual-cure resin-based materials.52,53 This is primarily due to acid deactivation of the aromatic tertiary amines that play a crucial role in chemical curing mechanisms of most of these materials.20 To overcome this issue, several universal adhesives, when used in conjunction with self- and dual-cure resin cements, require the addition of a separate “activator” (typically arylsulfinate salts) either all of the time (Prime&Bond Elect) or unless specific dedicated amine-free resin cements are used (Scotchbond Universal, Clearfil Universal). At least one universal adhesive (All-Bond Universal) is compatible with most common self- and dual-cure resin cements without the use of a separately applied activator. This is because this adhesive is less acidic, with a pH of 3.2, than other universal adhesives that have pHs ranging between 2.0 and 3.0. This is enough of a difference to allow the reactions necessary for chemical curing of self- and dual-cure resin cements and composites when using All-Bond Universal.54 Likewise, the two-component systems that are also marketed as universal adhesives (OptiBond XTR, Futurabond Universal) do not require the use of a separate activator, because the activator is already incorporated into one of the components of these systems. The manufacturers of some universal adhesives with pH less than 3.0 (Adhese® Universal, Ivoclar Vivadent; Prelude One) suggest that bonding to dual-cure resin cements is acceptable as long as the adhesive is light-cured first. In the author’s opinion the use of a universal adhesive with a pH less than 3.0 is risky if the dentist is relying on the self-cure mechanism of a dual-cure resin cement (which may be the case when light penetration is an issue) unless an additional activator is used.
Will Universal Adhesives
Replace Dedicated Primers?
The manufacturers of most universal adhesives state they can be used not only for bonding to dentin and enamel, but as adhesive primers on substrates such as zirconia, noble and non-precious metals, composites, and silica-based ceramics. The question isn’t whether universal adhesives are capable of bonding to these substrates (they are), but are they as effective, both initially and, more importantly, over time, as separately placed dedicated primers? There is some controversy surrounding this question. Opinions differ from manufacturer to manufacturer, and, in the author’s view, additional objective independent research is needed before making definitive recommendations.
In terms of bonding to zirconia, there are studies that demonstrate some universal adhesives are very effective as zirconia primers.51,55-57 However, at least two studies also found that the bond strength values of some universal adhesives to zirconia decreased significantly after thermocycling or 6 months of water storage.57,58 There are also respected chemists and researchers who feel that the most durable bond to zirconia is attained when separate and dedicated primers are employed.19 One such primer (Z-Prime), consisting of a phosphate ester (10-MDP) and carboxylic acid monomer (BPDM), has proven to be especially effective in this regard59-63 and does not require light-curing, which alleviates concerns some may have regarding film thickness (universal adhesives used as zirconia primers should be air-thinned and light-cured). Some self-etching 10-MDP-containing resin cements, such as Panavia SA Cement (Kuraray), have also shown promise bonding to both tooth tissues and substrates such as zirconia without the use of separately placed adhesives or primers.64 It is possible that products of this nature would perform even better if the zirconia surface were first treated with a universal adhesive or dedicated zirconia primer (the author is unaware of any research that has specifically examined this).
Another issue is how the dentist should treat the zirconia surface prior to placing a universal adhesive or dedicated zirconia primer. It is the author’s strong opinion that the zirconia surface should be sandblasted prior to utilizing any adhesive, primer, or resin-based cement. There is significant support in the literature for this recommendation.55,61-63,65-67 While there is some concern that sandblasting has the potential to induce surface and subsurface cracks and/or defects that could reduce physical properties,68,69 the author is unaware of any studies or anecdotal evidence that demonstrates this to be a clinical problem. Sandblasting zirconia is useful in terms of cleaning the target surface of impurities, increasing surface roughness, raising surface energy, improving the bond to primers and adhesives, and generally optimizing the surface prior to bonding or cementation. The author recommends using a sandblaster (eg, Microetcher™ II, Danville Materials) with 30-µm to 50-µm aluminum oxide (Al2O3) at 30 PSI to 40 PSI of air pressure (2.0 to 2.8 bar). The intaglio surface of the restoration is sandblasted after it has been tried in and just prior to dedicated primer or universal adhesive application. If the dentist does not have a sandblaster then the author recommends the dentist have the laboratory sandblast the restoration and then use a product such as Ivoclean (Ivoclar Vivadent), which is a solution of sodium hydroxide, polyethylene glycol, water, and zirconia oxide, after the restoration has been tried in and prior to primer application. While the use of phosphoric acid (H3PO4) can be an effective cleaning agent for saliva-contaminated silica-based ceramics (such as lithium disilicate)70 it is contraindicated for cleaning zirconia surfaces. This is because phosphate ions from the phosphoric acid remain bound to the zirconia surface (even after rinsing) and compete with phosphate ions from zirconia primers for reaction sites on the surface (zirconia has a strong affinity for phosphate ions). Likewise, the phosphate ions in saliva can tie-up reactive sites on the zirconia surface. Studies show that the best way to treat saliva-contaminated sandblasted zirconia surfaces is by re-sandblasting or using a strongly alkaline cleaning solution such as Ivoclean.71-73
Some universal adhesives also claim they can be used in lieu of dedicated silane coupling agents when bonding to silica-based ceramics (feldspathic, lithium disilicate, etc.). Historically, the use of hydrofluoric acid (HF) followed by the application of a dedicated silane coupling agent has been the treatment of choice in this regard, and the author has previously discussed this topic in great detail.74 Silanes are a class of organic molecules that contain one or more silicon atoms. Dozens of different silane compounds exist and are used extensively in industry and manufacturing. The silane typically used in dentistry, for both intraoral repair and the priming of silica-based ceramic restorations prior to placement, is 3-methacryloxypropyltrimethoxysilane (Figure 13 through Figure 16). The manufacturers of some universal adhesives have incorporated silane directly into their adhesive formulations, as noted in Table 1, with the idea that the universal adhesive can now be used instead of a separately applied dedicated silane solution. This makes sense as long as all the other chemistry found in universal adhesives does not interfere with silane stability and performance. The manufacturers of other universal adhesives have chosen not to add silane into their formulations because they question the stability of silane in the acidic environment of a universal adhesive and/or believe the chemical interaction of silane with silica-based ceramics is significantly inhibited when combined with all the other monomers found in universal adhesives. Indeed, contact angle studies have found that the incorporation of bis-GMA resin or MDP (both of which are used in universal adhesives) into silane solutions substantially reduced the priming efficiency and chemical interaction of the silane with silica-based lithium disilicate compared to pure silane controls.75,76 Once again, in the author’s opinion, the efficacy and wisdom of incorporating silane into universal adhesives is an area that requires further study and clarification. When bonding to silicate-based ceramics, the author’s personal preference, at least at this time, is to etch the porcelain with HF followed by the use of a dedicated pre-hydrolyzed silane primer (eg, RelyX™ Ceramic Primer, 3M ESPE; Porcelain Primer, Bisco, Inc.; Ultradent® Silane, Ultradent Products, Inc.) that is free of any added monomers or resins (pure silane).
Is It Time to Switch to a Universal Adhesive?
In August 2014, at a “key opinion leader” meeting comprised of 32 dentists, academicians, researchers, and chemists, the author conducted a written survey that included several questions,14 some of which, along with responses and attendee comments, are listed below:
Question: “Do you, or have you, used any of the new universal adhesive systems?” Of the 32 responses, 30 answered “yes.”
Question: “At this time what is your ‘go-to’ adhesive, ie, the one you use, or would recommend, most often?” There were 19 responses answering universal adhesives (two such products in particular were mentioned). Nine respondents answered either 4th or 5th generation total-etch systems, and four answered 6th generation self-etching systems.
Question: “Even though universal adhesives are supposed to bond to a variety of substrates, do you still feel separate dedicated metal, zirconia, and porcelain primers should be used? If yes, why do you feel that way?”
Most interestingly, 29 of the 32 respondents to the last question said they would still use a dedicated primer. Comments included: not trusting that universal adhesives would work as well as a separate primer; stability being an issue; Z-Prime Plus giving the best bond strength to zirconia; longer-range research data existed for dedicated primers; more steps still proves to be more durable, reliable, and longer lasting. Another concern was “never felt comfortable with silane being contaminated.” It should be pointed out that comments such as these are sometimes visceral in nature and not necessarily based on science. In any case, it is clear that there are some concerns about the ability of universal adhesives to perform as predictably as dedicated primers (especially when stressed by aging or thermocycling), and more research is needed that directly compares the two.
So, is it time to make the switch to a universal adhesive? Certainly, the versatility, simplicity, potential to reduce product inventory, chemistry, and relatively hydrophobic nature of polymerized universal adhesives do make them an attractive option. The author has been using a 10-MDP-containing universal adhesive system, most often in total- or selective-etching mode, almost exclusively now for just over 2 years with excellent clinical success. There are certain points to keep in mind that will optimize the performance of universal adhesive systems (as well as all adhesive systems):
1) Make sure to evaporate the solvents. All adhesive systems employ acetone, ethanol, water, or a combination of these as solvents for their particular monomers. It is extremely important to evaporate these solvents as completely as possible by air-drying for an adequate period of time prior to polymerization. Inadequate solvent evaporation has been associated with incomplete resin polymerization, nanoleakage, and decreased bond strength.77,78 Increasing the manufacturer’s recommended air-drying times and/or the use of warm air dryers may be prudent in this regard.
2) Even good chemistry will not overcome poor clinical technique. For example, all universal adhesives recommend “rubbing” or “scrubbing” the tooth preparation with adhesive for at least 20 seconds, drying, and then light-curing for an adequate period of time with a quality bonding light. Dentists should critically examine their own technique to make sure these guidelines are being followed.
3) Check the expiration date. All bonding agents utilize chemistries that can deteriorate significantly over time. This is especially true when they are subjected to high temperatures. Refrigeration may be useful in this regard, but adhesives should be removed and allowed to warm up to room temperature at least 30 minutes prior to use.79
4) Be sure to read the directions. All adhesive systems tend to have their own specific placement and handling idiosyncrasies that must be followed precisely for optimal results. What works well for one system may not be applicable for another.
Conclusion
Proper management of the adhesive interface is crucial for the predictable placement of many current dental restorations. This requires an understanding of the materials being utilized, the substrate being bonded to, and a correct and precise clinical protocol. It is incumbent on every dentist to learn about the specific adhesive system being used, its idiosyncrasies, strengths, and weaknesses, and how to maximize its performance. While clinical trials and clinical experience remains the ultimate test for all dental materials, universal adhesives represent an exciting and promising new class of dental adhesives that the author suspects will soon dominate the adhesive marketplace.
ACKNOWLEDGMENTS
During the preparation of this article the author spoke or corresponded with dozens of individuals from many different dental companies. He would especially like to thank the following individuals for their input, advice, and general support: Dr. Byoung Suh and Dr. Liang Chen (Bisco, Inc.); Dr. Joe Oxman and Jon Fundingsland (3M ESPE); Gregor Connell (VOCO Canada); Dr. Shashikant Singal (Ivoclar Vivadent); Dr. Xiangxu (Sean) Chen and Dr. Patrick Roetzer (Danville Materials); Dr. Xuejun (Eugene) Qiane (Kerr Corporation); and Dr. John Burgess (University of Alabama Birmingham).
Disclosure
The author has no affiliation with any of the companies mentioned in this article.
About the Author
Gary Alex, DMD
Private Practice, Huntington, New York
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Buonocore MG. A simple method of increasing the adhesion of acrylic filling to enamel surfaces. J Dent Res. 1955;34(6):849-853.
2. Buonocore MG, Matsui A, Gwinnett AJ. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch Oral Biol. 1968;13(1):61-70.
3. Alex G. Adhesive dentistry in the new millennium. Oral Health. 2000;59-64.
4. Gwinnett AJ. Bonding basics: What every clinician should know. Esthetic Dent Update. 1994;5:35-41.
5. Van Meerbeek B, Lambrechts P, Inokoshi S, et al. Factors affecting adhesion to mineralized tissues. Oper Dent. 1992;(suppl 5):111-124.
6. Gwinnett AJ. Smear layer: morphological considerations. Oper Dent Suppl. 1984;3:2-12.
7. Brännström M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984;3:35-42.
8. Pashley DH. Smear layer: overview of structure and function. Proc Finn Dent Soc. 1992;88(suppl 1):215-224.
9. Alex G. Is total-etch dead? Evidence suggests otherwise. Compend Contin Educ Dent. 2012;33(1):12-26.
10. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-273.
11. Fukegawa D, Hayakawa S, Yoshida Y, et al. Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res. 2006;85(10):941-944.
12. Van Landuyt KL, Yoshida Y, Hirata I, et al. Influence of the chemical structure of functional monomers on their adhesive performance. J Dent Res. 2008;87(8):757-761.
13. Key opinion leader symposium held at 3M ESPE; July 2014; Wonewok, MN.
14. Key opinion leader symposium held at Bisco, Inc.; August 2014; Chicago, IL.
15. Suh BI, Feng L, Pashley DH, Tay FR. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J Adhes Dent. 2003;5(4):267-282.
16. Nishiyama N, Tay FR, Fujita K, et al. Hydrolysis of functional monomers in single-bottle self-etching primer-correlation of 13C NMR and TEM findings. J Dent Res. 2006;85(5):422-426.
17. Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21(10):895-910.
18. Kadoma Y. Surface treatment agent for dental metals using a thiirane monomer and a phosphoric acid monomer. Dent Mater J. 2002;21(2):156-159.
19. Chen L, Suh BI, Brown D, Chen X. Bonding of primed zirconia ceramics: evidence of chemical bonding and improved bond strengths. Am J Dent. 2012;25(2):103-108.
20. Suh BI. Principles of Adhesive Dentistry: A Theoretical and Clinical Guide for Dentists. Newtown, PA: Aegis Publications LLC; 2013.
21. De Munck J, Van Meerbeek B, Yoshida Y, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82(2):136-140.
22. Hashimoto M, Ito S, Tay FR, et al. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;83(11):843-848.
23. Yoshida Y, Yoshihara K, Nagaoka N, et al. Self-assembled Nano-layering at the Adhesive interface. J Dent Res. 2012;91(4):376-381.
24. Yoshihara K, Yoshida Y, Hayakawa S, et al. Novel fluoro-carbon functional monomer for dental bonding. J Dent Res. 2014;93(2):189-194.
25. Z-PRIME Plus evaluation. The Dental Advisor. December 2010;27(10).
26. Tay FR, Pashley DH. Have dental resins become too hydrophilic? J Can Dent Assoc. 2003;69(11):726-731.
27. Tay FR, Pashley DH, Suh BI, et al. Single-step adhesives are permeable membranes. J Dent. 2002;30(7-8):371-382.
28. De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84(2):118-132.
29. Suh BI, Chen L, Brown DJ. A novel concept: the introduction of cross linking monomers into a self-etch adhesive to create a more hydrophobic and durable bond. Oral Health. 2011;March:62-66,94.
30. Van Landuyt KL, Snauwaert J, De Munck J, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28(26)3757-3785.
31. Nakaoki Y, Nikaido T, Pereira PN, et al. Dimensional changes of demineralized dentin treated with HEMA primers. Dent Mater. 2000;16(6):441-446.
32. Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dent Mater. 1992;8(2):125-130.
33. Jacobsen T, Soderholm KJ. Some effects of water on dentin bonding. Dent Mater. 1995;11(2):132-136.
34. Van Landuyt KL, De Munck J, Snauwaert J, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84(2):183-188.
35. Burrow MF, Inokoshi S, Tagami J. Water sorption of several bonding resins. Am J Dent. 1999;12(6):295-298.
36. Pashley EL, Zhang Y, Lockwood PE, et al. Effects of HEMA on water evaporation from water–HEMA mixtures. Dent Mater. 1998;14(1):6-10.
37. Van Meerbeek B, De Munck J, Yoshida Y, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003;28(3):215-235.
38. Van Meerbeek B, Yoshihara K, Yoshida Y, et al. State of the art of self-etch adhesives. Dent Mater. 2011;27(1):17-28.
39. Perdigao J, Lopes L, Lambrechts P, et al. Effects of a self-etching primer on enamel shear bond strengths and SEM morphology. Am J Dent. 1997;10(3):141-146.
40. Miyazaki M, Sato M, Onose H. Durability of enamel bond strength of simplified bonding systems. Oper Dent. 2000;25(2):75-80.
41. El-Askary FS, Anwar MN, Munoz MA, et al. Micro-shear bond strength of universal adhesives to uncut enamel [abstract]. J Dent Res. 2014;93(spec iss B). Abstract 1139.
42. Peumans M, De Munck J, Van Landuyt K, et al. Five-year clinical effectiveness of a two-step self-etching adhesive. J Adhes Dent. 2007;9(1):7-10.
43. Abdalla AI, El Sayed HY. Clinical evaluation of a self-etch adhesive in non-carious cervical lesions. Am J Dent. 2008;21(5):327-330.
44. Ermis RB, Temel UB, Cellik EU, Kam O. Clinical performance of a two-step self-etch adhesive with additional enamel etching in Class III cavities. Oper Dent. 2010;35(2):147-155.
45. Understanding the newest generation of adhesives: universal bonding agents. The Dental Advisor. March 2013;30(2).
46. Wagner A, Wendler M, Petschelt A, et al. Bonding performance of universal adhesives in different etching modes. J Dent. 2014;42(7):800-807.
47. Perdigao J, Sezinando A. Evaluation of a new universal adhesive using different bonding strategies [abstract]. J Dent Res. 2012;91(spec iss A). Abstract 18.
48. de Goes MF, Shinohara MS, Freitas MS. Performance of a new one-step multi-mode adhesive on etched vs non-etched enamel on bond strength and interfacial morphology. J Adhes Dent. 2014;16(3):243-250.
49. McLean D. Enamel Bond Strength of New Universal Adhesive Bonding Agents [master’s thesis]. Bethesda, MD: Uniformed Services University of the Health Sciences; 2013.
50. Perdigao J, Munoz MA, Luque-Martinez IV, et al. Enamel Etching Patterns of Universal Adhesives — FESEM Analyses [abstract]. J Dent Res. 2014;93(spec iss B). Abstract 474.
51. New Universal Adhesives (Part II): Faster, Easier, Better? Clinicians Report. 2012;5(8):1-3.
52. Suh BI, Feng L, Pashley DH, Tay FR. Factors contributing to the incompatibility between simplified-step adhesives and self-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J Adhes Dent. 2003;5(4):267-282.
53. Schittly E, Bouter D, Le Goff S, et al. Compatibility of five self-etching adhesive systems with two resin luting cements. J Adhes Dent. 2010;12(2):137-142.
54. Suh BI. Universal adhesives: The evolution of adhesive solutions continues. Compend Contin Educ Dent. 2014;35(4):278.
55. Amaral M, Belli R, Cesar PF, et al. The potential of novel primers and universal adhesives to bond to zirconia. J Dent. 2014;42(1):90-98.
56. Seabra B, Arantes-Oliveira S, Portugal J. Influence of multimode universal adhesives and zirconia primer application techniques on zirconia repair. J Prosthet Dent. 2014;112(2):182-187.
57. Kim JH, Chae SY, Lee Y, et al. Effects of multipurpose, universal adhesives on resin bonding to zirconia ceramic. Oper Dent. 2014 Aug 1. [Epub ahead of print]
58. de Souza G, Hennig D, Aggarwal A, Tam LE. The use of MDP-based materials for bonding to zirconia. J Prosthet Dent. 2014;112(4):895-902.
59. Kobes KG, Vandewalle KS. Bond strength of resin cements to zirconia conditioned with primers. Gen Dent. 2013;61(6):73-76.
60. Inokoshi M, Poitevin A, De Munck J, et al. Bonding effectiveness to different chemically pre-treated dental zirconia. Clin Oral Investig. 2014;18(7):1803-1812.
61. Yi YA, Ahn JS, Park YJ, et al. The effect of sandblasting and different primers on shear bond strength between yttria-tetragonal zirconia polycrystal ceramic and a self-adhesive resin cement. Oper Dent. 2014 Aug 1. [Epub ahead of print]
62. Barragan G, Chasqueira F, Arantes-Oliveria S, Portugal J. Ceramic repair: influence of chemical and mechanical surface conditioning on adhesion to zirconia. Oral Health Dent Manag. 2014;13(2)155-158.
63. Zandparsa R, Talua NA, Finkelman MD, Schaus SE. An in-vitro comparison of shear bond strength of zirconia to enamel using different surface treatments. J Prosthodont. 2014;23(2):117-123.
64. Koizumi H, Nakayama D, Komine F, et al. Bonding of resin-based luting cements to zirconia with and without the use of ceramic priming agents. J Adhes Dent. 2012;14(4):385-392.
65. Sahabi M, Yaghmaie K, Fayaz A. Effect of air-abrasion on microshear bond strength of two resin cements to Cercon porcelain. J Res Med Sci. 2010;4(3):142-145.
66. Yun JY, Ha SR, Lee JB, Kim SH. Effect of sandblasting and various metal primers on the shear bond strength of resin cement to Y-TZP ceramic. Dent Mater. 2010;26(7):650-658.
67. Wolfart M, Lehman F, Wolfart S, Kern M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater. 2007;23(1):45-50.
68. Zhang Y, Lawn BR, Rekow DE, Thompson VP. Effect of sandblasting on long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004;71(2):381-386.
69. Chintapalli RK, Marro FG, Jimenez-Pique E, Anglada M. Phase transformation and subsurface damage in 3Y-TZP after sandblasting. Dent Mater. 2013;29(5):566-572.
70. Klosa K, Wolfart S, Lehmann F, et al. The effect of storage conditions, contamination modes, and cleaning procedures on the resin bond strength too lithium disilicate ceramic. J Adhes Dent. 2009;11(2):127-135.
71. Yang B, Lange-Jansen HC, Scharnberg M, et al. Influence of saliva contamination on zirconia ceramic bonding. Dent Mater. 2008;24(4):508-513.
72. Chen L, Suh BI, Shen H. Minimize the contamination of zirconia restoration surface with saliva [abstract]. J Dent Res. 2013;92(spec iss A). Abstract 1654.
73. Feitosa SA, Patel D, Borges A, et al. Effect of cleaning methods on saliva-contaminated zirconia–An evaluation of resin bond durability. Oper Dent. 2014 Aug 19. [Epub ahead of print]
74. Alex G. Preparing porcelain surfaces for optimal bonding. Compend Contin Educ Dent. 2008;29(6):324-336.
75. Chen L, Shen H, Suh BI. Effect of incorporating BisGMA resin on the bonding properties of silane and zirconia primers. J Prosthet Dent. 2013;110(5):402-407.
76. Suh BI, Shah M, Chen L. Additional resin or acidic monomer inhibits silane primer [abstract]. J Dent Res. 2014;93(spec iss A). Abstract 792.
77. Hashimoto M, Tay FR, Svizero NR, et al. The effects of common errors on sealing ability of total-etch adhesives. Dent Mater. 2006;22(6):560-568.
78. Luque-Martinez IV, Perdigao J, Munoz MA, et al. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater. 2014;30(10):1126-1135.
79. Sundfeld RH, da Silva AM, Croll TP, et al. The effect of temperature on self-etching adhesive penetration. Compend Contin Educ Dent. 2006;27(10):552-557.