You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
In 2014, an estimated 14,000 fatalities were due to opioid overdoses in cases in which the drug was prescribed for therapeutic purposes. The overall incidence was more than 28,000 deaths for all opioid-related deaths (prescription and illicit).1 By comparison, the total opioid overdose death rate is higher than the annual US death rates from AIDS/HIV (6955)2 or oral cancer (>8000).3 Opioid prescribing by dentists is estimated to be 11% of the overall annual number of opioid prescriptions in the United States,4 indicating that approximately 1500 deaths annually may be attributable to unused opioids originally prescribed by dentists for therapeutic purposes. This number may be much higher as prescribed opioids are often reported as the “gateway” for heroin addiction.5 These projections suggest that dentists’ prescriptions for opioid analgesics may ultimately be associated with opioid overdose deaths. The overall burden is likely higher for management of acute dental pain as emergency department physicians also overwhelmingly prescribe opioid analgesics for nontraumatic dental conditions.6,7 The continued routine prescribing of opioid analgesics for acute dental pain represents an unwarranted clinical practice, as non-steroidal anti-inflammatory drugs (NSAIDs) are almost always more efficacious than opioid combinations (Figure 1), produce a lower incidence of adverse events, and do not contribute to drug abuse.
Below, we review the evidence that supports this conclusion and consider the role of patients’ expectations for receiving an opioid for acute dental pain that conflicts with the ethical imperative to “do no harm” for analgesic prescribing as part of dental care. We also provide alternative evidence-based therapeutic strategies for treating acute pain in dental practice. The data presented indicate opioids should be prescribed only as a last choice for acute dental pain. A failure for the dental profession to change from routine opioid prescribing for acute dental pain to more rational alternatives in the face of overwhelming evidence may continue to significantly contribute to the public health crisis in the opioid overdose epidemic.
Analgesic Drug Prescribing
The inadequate management of acute and chronic pain in the United States resulted in the implementation of Veterans Health Administration’s 1999 initiative “Pain as the 5th Vital Sign” for evaluating patients in medical environments and the much more recent 2016 National Pain Strategy8 to improve pain management and research in the United States. Recognition of the pain-relieving effects of antidepressant and anticonvulsant drugs for patients with chronic pain did result in the development of more effective drugs for neuropathic pain. The much-heralded advantages of cyclo-oxygenase-2 (COX-2) selective inhibitors for both acute and chronic pain were largely unfilled by belated recognition of cardiovascular toxicity, which led to withdrawal of most COX-2 inhibitors from the market (except celecoxib). The increased emphasis on better pain management may have resulted in dramatic increases in opioid prescribing but may also have contributed to widespread opioid misuse, abuse, and diversion, leading to dramatic increases in opioid overdose deaths. The total number of opioid pain relievers, such as hydrocodone and oxycodone, prescribed in the United States has increased drastically from 76 million in 1991 to nearly 207 million in 2013.9 The rate of overdose deaths due to prescription pain medications has more than tripled in the past 20 years.9 Moreover, the increased availability of illicit heroin now provides a more cost-effective way of sustaining opioid abuse once it has been established.9
Opioid analgesics are effective for relieving pain by acting at opiate receptors in the central nervous system and can be safely administered for acute and chronic pain under supervision of a health care practitioner. Repeated administration of an opioid drug eventually produces cellular changes that result in the need for increasing doses of the drug to produce the same effect (tolerance). Continued administration also results in molecular changes that require continuous receptor occupancy to avoid the unpleasantness of withdrawal (physical dependence) and an intense craving for the euphoria associated with opioid drugs (psychological dependence). These effects can be mitigated by administration of drugs in a therapeutic context. When withdrawal and dependence occur as part of drug abuse, the intense craving for opioid drugs promotes attempts to acquire drugs illicitly, which is referred to as addiction. Opioid drug abuse is partially heritable and can be precipitated from as little as a single dose to the progressive continuum of tolerance, dependence, and eventual addiction and overdose.
Variability in the composition and potency of illicit opioids increases the probability of inadvertent overdose,9 which, if not witnessed, can progress to respiratory depression, unconsciousness, and death. Rehabilitation from opioid dependence has had limited success while substitution of illicit opioids with methadone-therapy maintenance minimizes cravings and drug-seeking behavior but does not directly address the underlying physical and psychological dependence. Distribution of naloxone kits to emergency-medical technicians and families of drug-dependent individuals has improved resuscitation success but also provides a rationale for continued abuse on the assumption that respiratory depression can be readily reversed.10 Any opioid prescription for acute pain can progress to lifelong drug dependence or acute mortality due to drug overdose, if precautions are not taken.
In theory, opioid prescribing for acute pain contributes to greater availability of opioid drugs available for misuse, abuse, or diversion, thereby indirectly adding to the serious morbidity and mortality associated with prescription opioids. An estimated 12% of patients reporting back pain, dental pain, or headache in emergency departments are “doctor shopping” for an opioid prescription.11 Similarly, a survey of patients seeking care at an emergency dental clinic indicated that 37% of patients reported nonmedical use of prescription pain medications in the previous 30 days.12 Among factors contributing to the initiation of substance use, nonmedical use of prescription pain medications was the second most-prevalent illicit first-time drugs for persons (>12 years old) in the United States.13 The connection with dentistry is concerning. The prevalence of dental practices and pharmacies in counties within Indiana was identified as the leading predictor of enrollment in drug-abuse treatment programs in the state.14 This observation suggests that the availability of prescription opioids in a community (eg, unused medications in a household cabinet) is associated with higher rates of opioid abuse and that management of acute orofacial pain may be contributing to dependence rates. Prescription monitoring programs play a role in helping to reduce opioid diversion, but these systems cannot readily differentiate analgesic prescribing for an appropriate clinical indication from prescribing that is not evidence based, such as prescribing an opioid rather than an NSAID for acute inflammatory pain.
Acute Dental Pain Management
Pain following a typical dental procedure such as a simple extraction is usually minor and transient and can be effectively managed with over-the-counter (OTC) drugs. When postprocedural pain follows a surgical extraction, implant placement, or endodontic procedure or when preexisting infection or inflammation has sensitized nociceptive processes, an analgesic with greater efficacy than OTC medications may be required. Analgesics for acute dental pain are usually orally administered, should produce minimal adverse events in ambulatory patients, and have minimal effects on cognition and alertness so that patients can return to the activities of daily living. These medications should be prescribed for a short period. Control of pain following a procedure that produces tissue injury and subsequent inflammation is best accomplished by targeting the etiology of the pain, ideally by attenuating the acute inflammatory process. Symptom management by interfering with the perception of pain with opioid drugs that act in the central nervous system does not target the cause of acute dental pain and often impairs alertness, cognition, and psychomotor function. In addition, ambulatory patients are more prone to adverse effects associated with motion, such as dizziness, nausea, and vomiting.15
Before NSAIDs were introduced in the mid 1970s to the US market, orally administered analgesics were a compromise between the ceiling of analgesic efficacy associated with aspirin and acetaminophen and the undesirable effects of opioids when given to ambulatory patients. A standard dose of a peripherally acting agent (aspirin or acetaminophen) was combined with an orally effective opioid (codeine or oxycodone) at a dose selected to provide additive analgesia, often combined with adjunctive agents thought to potentiate analgesia (caffeine) or to counter the adverse effects of the opioid (promethazine). A critical appraisal of analgesic combinations by the Food and Drug Administration resulted in elimination of several adjuncts and reformulation of some combinations to meet regulatory requirements for safety and additive analgesia.16 The treatment of acute pain was subsequently revolutionized by the introduction of NSAIDs and the development of the oral-surgery model17 for evaluating analgesic efficacy and side-effect liability for dental outpatients, rather than relying on extrapolation from cancer or general-surgery studies.18 Drugs and doses now used for acute postprocedural pain have been characterized through thousands of well-controlled clinical trials, providing a large and robust evidentiary basis for therapeutic recommendations.
NSAID Analgesics
NSAIDs are widely used for acute dental pain as they are generally more efficacious than aspirin, acetaminophen, or codeine (Figure 1) due to the inflammatory cause of most dental pain and NSAIDs’ prominent anti-inflammatory effects. NSAID therapy is also preferable for ambulatory patients who generally experience a high incidence of side effects when given an opioid. NSAIDs modestly suppress the development of edema after surgical procedures,19 providing additional benefit without the potential liabilities of administering steroids. When used as directed, OTC dosing regimens for ibuprofen, ketoprofen, or naproxen sodium are safe and effective across a wide variety of dental-pain conditions.20 These conditions and the over 40 years of clinical experience with ibuprofen make NSAIDs the drug class of choice for dental pain for patients who do not have any contraindications to its use (Table 1).
Ibuprofen at a dose of 400 mg is superior to 650 mg of aspirin, 600 mg to 1000 mg of acetaminophen, combinations of aspirin and acetaminophen plus 60 mg of codeine, and 30 mg of dihydrocodeine when evaluated in the oral-surgery model.21-23 Administration of doses greater than 400 mg is not likely to result in greater peak analgesia, but the increased blood levels may modestly prolong the duration of effect.24 Other NSAIDs such as meclofenamate, naproxen sodium, and diclofenac result in analgesia that is comparable to ibuprofen, depending on the doses of each agent used. The use of NSAIDs also results in clinically significant analgesia for periodontal surgery,25 orthodontic tooth movement,26 and pain following endodontic procedures.27
Ketorolac tromethamine is an NSAID approved for parenteral and nasal administration for short-term management of moderate-to-severe pain, and several analgesic models have shown it is comparable in efficacy to 100 mg of meperidine and 10 mg to 12 mg of morphine.28,29 Parenteral administration of ketorolac produces less drowsiness, nausea, and vomiting than morphine;30 however, this route of administration limits its use for ambulatory patients to the initial dose prior to discharge. The ability of injectable ketorolac to overcome the slower onset of orally administered drugs, combined with analgesic efficacy comparable to that of parenteral opioids but with reduced side effects suggests that its use for a procedure likely to result in moderate-to-severe pain obviates the need to prescribe an opioid combination. The intravenous administration of ibuprofen or acetaminophen perioperatively may also minimize the need for the routine use of postoperative opioid pain relievers.
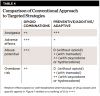
When an NSAID is combined with therapeutic strategies for preventing acute pain and minimizing its intensity, as described elsewhere,31 the rationale for prescribing an opioid combination to be taken as needed for acute pain is even less warranted. Table 2 summarizes a stepwise, multimodal approach to acute pain management that targets the inflammatory etiology of pain with NSAID administration to minimize the effects of inflammatory mediators released due to tissue injury and gene expression. Preventive administration of an NSAID and long-acting local anesthetic also blocks physiologic events that augment the effects of the inflammatory process. Adjunctive administration of acetaminophen provides additive analgesia without the deleterious effects of abuse-prone opioids such as oxycodone and hydrocodone. Prescribing tramadol is less likely to contribute to abuse, especially if a limited 3-day supply is prescribed without any refills. Administration of an opioid combination containing oxycodone or hydrocodone does not target inflammation, does not implement preventive strategies, and facilitates opioid misuse, abuse, and diversion that can lead directly to opioid overdoses.
Opioid Combination Analgesics
Many opioid analgesics have poor oral potency due to low oral bioavailability; opioids such as morphine, meperidine, and oxymorphone have such low oral potency that they are of little use in routine oral analgesic therapy.32 Notable exceptions are hydrocodone and oxycodone that are absorbed into the portal system and have much smaller first-pass metabolism by the liver. Codeine has better oral efficacy because there is significantly less loss due to first-pass metabolism and much is demethylated into morphine by the liver.
Another general problem with the opioid analgesics is their relatively high incidence of nausea and central nervous system (CNS) depression, which become more intense as the dosage is increased. Mild CNS depression manifested as sedation may sometimes be useful, but ambulatory dental patients generally want to be able to function normally after they leave the dental office. As a consequence, single-entity opioid analgesics are not the drugs of choice for the management of acute dental pain in ambulatory patients.
Codeine is commonly used in combination analgesics. Its effective oral-dose range is 30 mg to 90 mg, with 30 mg providing only minimal additive analgesia, 60 mg providing detectable analgesia with considerably more nausea and sedation, and 90 mg approaching the dose at which intolerable side effects appear.33 Codeine is available in combination with aspirin or acetaminophen. For most patients, 600 mg to 650 mg of either drug combined with 60 mg of codeine should provide some additive pain relief but with delayed and variable effects due to time needed for metabolism to morphine and genetic variability in this metabolic pathway. A combination of 400 mg of ibuprofen plus 60 mg codeine in comparison with 400 mg of ibuprofen alone results in modest, if any, additive analgesia for 1 to 2 hours.34,35 As a consequence of the weak evidence of therapeutic benefit in controlled clinical trials, a combination of these two drugs has never been approved for marketing in the United States.
Analgesic combinations containing oxycodone are generally perceived as being more effective than codeine-containing combinations based on the 10-fold to 12-fold greater potency attributed to oral oxycodone in comparison to oral codeine.18 However, the recommended dose of oxycodone in combination with aspirin or acetaminophen is 5 mg every 6 hours and should result in the same analgesia as 50 mg to 60 mg of codeine.36 Combinations of oxycodone with aspirin or acetaminophen result in greater analgesia that either drugs have alone, but are no more effective than an NSAID alone, while resulting in significantly higher incidence of adverse events in ambulatory patients.37 The results of several clinical trials and a systematic review indicate that 5 mg of oxycodone produces additive analgesia when combined with 400 mg of ibuprofen.38 Combining a 10-mg dose of oxycodone with 400 mg of ibuprofen produces additive analgesia but with a dose-related increase in drowsiness, nausea, and vomiting.37
Hydrocodone/acetaminophen combinations are the most widely prescribed analgesics in the United States but no published data have indicated that they result in analgesia that is comparable to a prescription dose of an NSAID.39 A formulation combining ibuprofen 200 mg with hydrocodone 7.5 mg is also marketed, but the 200-mg ibuprofen dose is suboptimal and no evidence suggests that the analgesia provided by this combination is greater than 400 mg to 600 mg of ibuprofen alone. In patients with severe pain, combining one tablet of marketed fixed-dose combination with 200 mg to 400 mg of ibuprofen for a combined dose 400 mg to 600 mg of ibuprofen with 7.5 mg of hydrocodone should produce greater analgesia (and side effects) than an NSAID alone.
Patients’ Expectations and Opioids
Although local anesthesia effectively controls procedural pain, postoperative pain is driven by expression of inflammatory mediators for 2 to 3 days following a procedure and varies highly in intensity across patients due to a wide variety of influences. Our still-limited understanding of how these factors work together is inadequate for predicting, with certainty, the intensity of the patient’s postoperative pain. The usual approach is to select an appropriate analgesic regimen based on estimated pain intensity due to the extent of tissue injury, the patient’s medical history, the etiology of the nociceptive input (eg, inflammation), the patient’s self-report of past pain experiences, and the available choice of analgesic drugs. Patients’ expectations for a comfortable postoperative recovery with minimal impact on their activities of daily living are generally biased toward receiving the most efficacious drug. However, patients do not take into account the risk for common adverse events, possible idiosyncratic reactions when taking a drug for the first time or when administered in combination with another drug, the personal impact of opioid misuse and abuse, or the societal impact of drug dependence and drug-seeking behavior.
The possible discrepancy between wide inter-individual variability in pain and patients’ expectations for minimal postoperative discomfort presents a therapeutic dilemma. For some patients, the analgesic efficacy is adequate and the incidence of adverse events minimal. However, for those with high-intensity pain, analgesic efficacy is inadequate, and for others the side-effect liability is unacceptable. The potential for exposure to opioids prescribed for therapeutic purposes leading to drug misuse and possible drug dependence is also variable and unpredictable. Given the likely discrepancy between patients’ expectations and the need to balance analgesic efficacy with side-effect liability and potential for adding to the mortality associated with opioid overdose, alternative therapeutic strategies are needed. These include individualizing the analgesic drug regimen, using interventions that attempt to prevent pain rather than “manage” it after onset, and educating patients and their significant others about risks for opioid use, especially when administered to adolescents who are more vulnerable to drug abuse. Table 2 provides suggestions to minimize opioid misuse or diversion that can be readily implemented before considering whether to prescribe an opioid combination analgesic.
Alternatives for Acute Dental Pain
Rather than routinely administering a fixed-dose opioid combination analgesic for all patients who are expected to have moderate-to-severe pain, a flexible analgesic strategy is recommended to optimize NSAID analgesia. This may also help to minimize opioid-induced adverse events and reduce the risk for individual patients being exposed to the risk for opioid abuse, leading to possible dependence and opioid overdose death (Table 3 and Table 4). Levels of pain that might be expected after a simple extraction or endodontic treatment to a tooth that was asymptomatic prior to treatment may be effectively managed with an OTC (mild pain) medication or a prescription dose of an NSAID such as ibuprofen, ketoprofen, or naproxen. The analgesic can be administered prior to or immediately following the procedure to delay the onset of postoperative pain, minimize its intensity following the offset of local anesthesia, and attenuate the development of sensitization leading to hyperalgesia over the 48 to 72 hours following a procedure that produces tissue injury.
For a surgical procedure such as removal of impacted third molars, implant placement, and endodontic treatment of a tooth that has already been sensitized due to subacute inflammation, preventive analgesia should be initiated with use of a long-acting local anesthesia and NSAID administration prior to or immediately following the procedure. This is to suppress the formation of prostaglandin E2 that promotes postoperative pain, swelling, and the development of sensitization leading to hyperalgesia. The NSAID should be continued for 48 to 72 hours based on the dosing interval for the NSAID prescribed. Acetaminophen 600 mg to 650 mg can be co-administered to provide additive analgesia with minimal additional side effects, either at the same time as the NSAID is administered or alternating as long as the maximum recommended dose for each drug is not exceeded. Patients can be provided with a prescription for an opioid combination drug to be filled only if pain is not adequately suppressed by the preventive regimen. Ultracet® combines acetaminophen with tramadol, which is an orally effective opioid drug.40-43
Ultracet has much less potential for misuse than an oxycodone or hydrocodone combination. Prescribe only a 3-day supply of the Ultracet, and provide the patient and a significant other or caregiver with instructions to dispose of the reminder of the prescription when no longer needed and to not share the medication with friends or relatives (Table 2). If severe pain persists beyond 3 days, the patient should be examined for possible postoperative complications, eg, infection or alveolitis, rather than be provided with another opioid-containing prescription.
Translating Evidence, Ethics, and Education
Procedures that warrant postoperative analgesic use will likely result in less discomfort for the patient and fewer side effects by shifting from traditional aspirin-opioid or acetaminophen-opioid combination to a preventive strategy. Combining two preventive regimens—the use of preoperative NSAID and long-acting local anesthetic—delays the onset of pain on the day of the procedure, lessens the intensity of pain when if it does occur, and attenuates the development of sensitization leading to hyperalgesia over the 48 to 72 hours following a tissue injury-producing procedure. Due to the wide variability in pain and analgesia that exists throughout the patient population, some individuals will report pain that requires intervention. Recommending the administration of acetaminophen in addition of an NSAID results in additive analgesia without opioid-mediated side effects and without exposing the patient to a drug that can be the first step in the development of misuse, with the possible catastrophic consequence of drug dependence. In the minority of cases, not as a routine practice, providing a prescription for an opioid combination that can be filled and administered only if needed provides additional pain relief for individual patients without exposing the population of patients to the risks for opioid abuse and decreases the supply of opioids available for possible diversion. This overall approach permits individualization of analgesic therapy within the limits of currently available medications in an outpatient setting and fulfills the ethical imperative to “do no harm” in the doctor-patient interaction and at the societal level.
Disclosure
The authors had no disclosures to report.
About the Authors
Raymond A. Dionne, DDS, PhD
Professor
Brody School of Medicine
Department of Pharmacology and Toxicology
East Carolina University
Greenville, North Carolina
School of Dental Medicine
Department of Foundational Sciences
East Carolina University
Greenville, North Carolina
Sharon M. Gordon, DDS, MPH, PhD
Associate Dean for Research Chair
School of Dental Medicine
Department of Foundational Sciences
Greenville, North Carolina
Paul A. Moore, DMD, PhD, MPH
Professor
School of Dental Medicine
Department of Dental Public Health
University of Pittsburgh School of Dental Medicine
Pittsburgh, Pennsylvania
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths–United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
2. AIDS and HIV. National Center for Health Statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/fastats/aids-hiv.htm. Accessed May 11, 2016.
3. Oral Cancer. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/OralHealth/oral_cancer/index.htm. Accessed May 11, 2016.
4. Tufts Health Care Institute Program on Opioid Risk Management. Executive summary: The role of dentists in preventing opioid abuse. 12th Summit Meeting. March 11-12, 2010. http://opioidriskmanagement.com/opioid/mar10docs/executivesummary.pdf. Accessed May 11, 2016.
5. Muhuri PK, Gfroerer JC, Davies MC. Association of nonmedical pain reliever use and initiation of heroin use in the United States. US Substance Abuse and Mental Health Services Administration. CBHSQ Data Review. August 2013. http://www.samhsa.gov/data/sites/default/files/DR006/nonmedical-pain-reliever-use-2013.htm. Accessed May 12, 2016.
6. Okunseri C, Okunseri E, Thorpe JM, et al. Medications prescribed in emergency departments for nontraumatic dental condition visits in the United States. Med Care. 2012;50(6):508-512.
7. Okunseri C, Dionne RA, Gordon SM, et al. Prescription of opioid analgesics for nontraumatic dental conditions in emergency departments. Drug Alcohol Depend. 2015;156:261-266.
8. Interagency Pain Research Coordinating Committee, National Institutes of Health. National Pain Strategy – A Comprehensive Population Health-Level Strategy for Pain. 2016. https://iprcc.nih.gov/docs/drafthhsnationalpainstrategy.pdf. Accessed May 12, 2016.
9. Volkow ND. Senate Caucus on International Narcotics Control. National Institute on Drug Abuse, National Institutes of Health. America’s addiction to opioids: heroin and prescription drug abuse. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse. May 14, 2014. Accessed May 12, 2016.
10. Becker D. National Public Radio. Reversing opioid overdoses saves lives but isn’t a cure-all. http://www.npr.org/sections/health-shots/2015/10/07/445399564/reversing-opioid-overdoses-saves-lives-but-isnt-a-cure-all. October 7, 2015. Accessed May 12, 2016.
11. Weiner SG, Griggs CA, Langlois BK, et al. Characteristics of emergency department “doctor shoppers.” J Emerg Med. 2015;48(4):424-431.
12. Ashrafiioun L, Edwards PC, Bohnert AS, Ilgen MA. Nonmedical use of pain medications in dental patients. Am J Drug Alcohol Abuse. 2014;40(4):312-316.
13. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication no. (SMA) 14-4863. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2013.
14. Wright ER, Kooreman HE, Greene MS, et al. The iatrogenic epidemic of prescription drug abuse: county-level determinants of opioid availability and abuse. Drugs Alcohol Depend. 2014;138:209-215.
15. Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesic for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;10:CD011407.
16. Rettig RA, Earley LE, Merrill RA, eds. Food and Drug Administration Advisory Committees. Committee to study the use of advisory committees by the Food and Drug Administration. Division of Health Care Policy. Institute of Medicine. Washington, DC: National Academy Press, 1992. http://www.nap.edu/read/2073/chapter/1#iv. Accessed May 13, 2016.
17. Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Therap. 1976;20(2):241-250.
18. Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. comparison of oral with intramuscular codeine and of oral with intramuscular oxycodone. J Pharmacol Exp Ther. 1978;207(1):92-100.
19. Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of nonsteroidal anti-inflammatory drugs, ibuprofen and flurbiprofen, with methylprednisolone and placebo for acute pain, swelling, and trismus. J Oral Maxillofac Surg. 1990;48(9):945-952.
20. Doyle G, Jayawardena S , Ashraf E, Cooper SA. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol. 2002;42(8):912-919.
21. Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. J Oral Surg. 1977;35(11):898-903.
22. Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy. 1989;10(6, pt 2):94s-105s.
23. Jain AK, Ryan JR, McMahon G, et al. Analgesic efficacy of low-dose ibuprofen in dental extraction. Pharmacotherapy. 1986;6(6):318-322.
24. Laska EM, Sunshine A, Marrero I, et al. The correlation between blood levels of ibuprofen and clinical response. Clin Pharmacol Therap. 1986;40(1):1-7.
25. Vogel RI, Desjardins PJ, Major KV. Comparison of presurgical and immediate postsurgical ibuprofen on postoperative periodontal pain. J Peridontol. 1992;63(11):914-918.
26. Ngan P, Wilson S, Shanfield J, Amini A. The effect of ibuprofen on the level of discomfort in patients undergoing orthodontic treatment. Am J Orthod Dentofacial Orthop. 1994;106(1):88-95.
27. Flath RK, Hicks HL, Dionne RA, Pelleu GB Jr. Pain suppression after pulpectomy with preoperative flurbiprofen. J Endodontics. 1987;13(7):339-347.
28. Fricke JR Jr, Angelocci D, Fox K, et al. Comparison of the efficacy and safety of ketorolac and meperidine in the relief of dental pain. J Clin Pharmacol. 1992;32(4):376-384.
29. O’Hara DA, Fragen RJ, Kinzer M, Pemberton D. Ketorolac tromethamine as compared with morphine sulfate for treatment of postoperative pain. Clin Pharmacol Ther. 1987;41(5):556-561.
30. Spindler JS, Mehlisch D, Brown CR. Intramuscular ketorolac and morphine in the treatment of moderate to severe pain after major surgery. Pharmacotherapy. 1990;10(6, pt 2):51s-58s.
31. Dionne RA, Gordon SM. Changing paradigms for acute dental pain: prevention is better than PRN. J Calif Dent Assoc. 2015;43(11):655-662.
32. Yaksh TL, Wallace MS. Opioids, analgesia and pain management. In: Brunton L, Chabner B, Knollman B, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill. 2011:499-501.
33. Haas D. Opioid analgesics and antagonists. In: Dionne RA, Phero JC, Becker DE, eds. Management of Pain & Anxiety in the Dental Office. Philadelphia, PA: WB Saunders. 2002:122.
34. Cooper SA, Engel J, Ladov M, et al. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy. 1982;2(3):162-167.
35. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521-527.
36. Petersen JK, Hansson F, Strid S. The effect of an ibuprofen-codeine combination in the treatment of patients with pain after removal of lower third molars. J Oral Maxillofac Surg. 1993;51(6):637-640.
37. Dionne RA. Additive analgesic effects of oxycodone and ibuprofen in the oral surgery model. J Oral Maxillofac Surg. 1999;57(6):673-678.
38. Derry S, Derry CJ, Moore RA. Single dose oral ibuprofen plus oxycodone for acute postoperative pain in adults. Cochrane Database Syst Rev. 2013;6:CD010289.
39. Moore PA, Hersh EV, Dionne RA, Cooper SA. Why do we prescribe Vicodin? J Am Dent Assoc. Accepted for publication 2016.
40. Sawaddiruck P. Tramadol hydrochloride/acetaminophen combination for the relief of acute pain. Drug Today (Barc). 2011;47(10):763-772.
41. McQuay HJ, Moore RA, Berta A, et al. Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroscopy. Brit J Anaesth. 2016;116(2):269-276.
42. Lasko B, Levitt RJ, Rainsford KD, et al. Extended-release tramadol/paracetamol in moderate-to-severe pain: a randomized, placebo-controlled study in patients with acute low back pain. Curr Med Res Opin. 2012;28(5):847-857.
43. Moore PA, Crout RJ, Jackson DL, et al. Tramadol hydrochloride: analgesic efficacy compared to codeine, aspirin with codeine and placebo after dental extraction pain. J Clin Pharm. 1998;38(6):554-560.