You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Sleep-related breathing disorders (SRBDs), including obstructive sleep apnea (OSA) and upper airway resistance syndrome (UARS), are characterized by decreased pressure that results in recurrent partial to complete collapse of the pharyngeal airway during sleep and are increasingly being recognized as a major public health problem because they affect at least one-third of the adult population.1,2 Although SRBDs are medical conditions, dentistry is uniquely positioned to screen, treat, and even prevent these chronic diseases with oral appliances, orthodontic treatment, and oral surgical procedures. In fact, in 2018, the American Dental Association adopted a policy statement mandating that dentists provide screening and treatment for SRBDs among children and adults.3
Historically, treatment has been determined by a diagnosis that is based on the number of airway events that occur during an hour of sleep. However, there is growing interest in determining which mechanisms represent the root causes of these airway challenges. Patients with similar disease severity index scores (ie, apnea-hypopnea index [AHI]) may have entirely different causes and therefore, could benefit from customized therapies that target the specific causal "traits." The variability among patient responses to anatomic-directed therapies is evidence that we need to do more. Appreciating this heterogeneity of the causes and consequences of SRBDs, it is no longer desirable or appropriate to treat based on disease severity index scores alone.
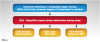
Pharyngeal anatomic impairment in patients with SRBDs can be modest, and because as many as 50% of OSA patients are non-obese, approximately 20% of patients with SRBDs have a level of upper airway impairment that is no different than that of people without SRBDs.4 Thus, for many patients, nonanatomic factors, or "traits," that modulate pharyngeal patency are crucial determinants in the development of an SRBD.5 In fact, recognizing that SRBDs are caused by the interplay between a variety of physiologic (ie, nonanatomic) variables and anatomic pharyngeal collapsibility, it can be appreciated that the diagnosis, management, and treatment of SRBDs requires a deeper understanding of the pathophysiology of the underlying disease (Figure 1).
Dentists have been anatomically phenotyping patients by identifying structural deficiencies associated with SRBDs, such as high body mass index, retrognathic mandible, maxillary hypoplasia, high-arched palate, obstructed nasal passageways, tongue position, hyoid bone level, and various cephalometric and cone-beam computed tomography data points.6 Beyond anatomic variables, which are the biggest causal factors of SRBDs, the literature reveals that approximately 69% of affected patients have at least one nonanatomic trait that contributes a variable influence to their airway function.5,7 These physiologic traits include compensatory neuromuscular responses, such as arousal threshold, loop gain, and muscle recruitment. The nonanatomic contributors become less significant as the anatomy becomes more deficient and more significant as the anatomy becomes closer to normalized.
Previously available treatments only targeted a single trait; however, data quantifying these airway challenges can now be extracted from standard overnight sleep studies, which helps clinicians efficiently select between appropriate therapies to arrive at the most successful treatment. A clear understanding of each of the trait targets, how the data is collected, and which treatments show promise is the basis of precision treatment.
Pharyngeal Critical Closing Pressure
Pharyngeal collapsibility refers to how likely the pharynx is to collapse at a given pressure. The gold standard measurement of pharyngeal collapsibility is called the pharyngeal critical closing pressure (Pcrit), which is measured in cm H2O (Figure 2). Pcrit is an objective measurement of upper airway collapsibility-an important pathogenetic factor in OSA. It is desirable to have a Pcrit that is very low (ie, a negative number). When a patient's Pcrit is "reduced" or "lowered," it means that it has become a negative number of greater magnitude, and therefore, a larger pressure drop is required to collapse the airway. The higher the Pcrit value (ie, a negative number with lesser magnitude or a positive number), the more easily the pharynx will collapse. The lower the Pcrit value, the better.
When atmospheric pressure is 0 cm H2O, a normal, healthy airway can withstand a pressure of -8 to -10 cm H2O before it collapses. It is normal to have pharyngeal pressure drop during inspiration. If a person's Pcrit increases above -8 cm H2O (ie, to a negative number of lesser magnitude or a positive number) and airway pressures fall below Pcrit during inspiration (ie, typically -8 to -10 cm H2O), the airway can collapse.8,9,10
Apneic patients tend to have a Pcrit above 0 cm H2O, and patients who predominantly experience hypopneas usually have a Pcrit of 0 to -4 cm H2O. Generally, individuals who snore will have a Pcrit of -4 to -6 cm H2O, whereas UARS patients have a Pcrit of -2 to -6 cm H2O.8,9,10,11
Effective therapy requires a lowering of the patient's Pcrit to below whatever the airway pressure maximally drops to during inspiration. Therefore, clinicians need to quantify the baseline Pcrit in order to make rational treatment choices. A Pcrit above -5 cm H2O reflects anatomic compromise. Anatomic-directed therapies, including surgical intervention, continuous positive airway pressure (CPAP) therapy, oral appliance therapy, weight loss, and positional therapy all have an impact on a patient's Pcrit value. Selecting and combining therapeutic choices that lower the Pcrit to at least -8 to -10 cm H2O will maintain a patent airway.5,8
Changing from sleeping with the mouth open to sleeping with the mouth closed lowers Pcrit by approximately 4 cm H2O.12 A 10% weight loss can lower Pcrit by 2 to 4 cm H2O, whereas a 17% weight loss can lower it by 7.5 cm H2O. In combination, mouth taping and minimal weight loss can lower Pcrit by 6 cm H2O. Oral appliance therapy can decrease a patient's Pcrit by 2.3 to 4 cm H2O, upper airway surgery can decrease it by approximately 3.3 cm H2O, and positional therapy (eg, supine avoidance) can decrease it by 2.2 cm H2O. In addition, surfactants may decrease Pcrit by 1.75 cm H2O.5
Algorithms have recently been developed that enable flow data from a standard polysomnogram (PSG) to be used to directly quantify Pcrit values without a catheter.9,13
Arousal Threshold
During sleep, the respiratory centers in the brain stem track mechanical constraints (eg, low lung volumes, pharyngeal negative pressure, resistance to airflow) and gas exchange abnormalities (eg, oxygen, pH, carbon dioxide changes), which can trigger arousal. To respond to these derangements in mechanics or gas exchange, a specific threshold of increased respiratory effort is associated with the occurrence of an arousal (Figure 3).
The respiratory arousal threshold is defined as the intrathoracic pressure level at which a given individual has an arousal. Some patients have a high arousal threshold (ie, they sleep through major stimuli), and other patients have a low arousal threshold (ie, they wake up easily). The respiratory arousal threshold is the nadir pressure immediately prior to cortical arousal.
Repetitive arousals perpetuate disturbances in the levels of blood gases and cause sleep fragmentation and deprivation. They also promote cyclical breathing and prevent the establishment and maintenance of more stable, deeper stages of sleep. Most importantly, the change from being asleep to being awake increases basal chemoreflex drive and sensitivity. Arousals from obstructive events can result in a greater degree of hyperventilation that, in turn, can lead to hypocapnia and a reduction in ventilatory drive, including the drive to upper airway dilator muscles. In this manner, arousals may perpetuate successive obstructions. The surges of sympathetic neural activity and fragmented sleep associated with arousals can also contribute to cardiovascular disease, metabolic disorders, and neurocognitive deficits.14 Strategies to reduce the incidence of arousals in these patients may allow for more stable breathing during sleep.
Although most respiratory events are associated with a cortical arousal, and arousals have been considered crucial to reopen the upper airway following a respiratory event in OSA, airway reopening can occur without an arousal as a result of other physiologic functions.
Roughly one-third of OSA patients have a low arousal threshold and may be candidates for targeted therapy.10 Patients whose OSA is predominantly associated with non-rapid eye movement sleep have greater decreases in ventilation and dynamic changes of PaCO2 during the transition from being awake to this stage of sleep. This finding may be helpful in identifying the clinical subgroup that should undergo treatment to stabilize ventilatory control by raising arousal threshold.15
A pharmacologic approach to raising the arousal threshold has been suggested for the low arousal threshold subgroup. Certain medications can be effective in raising the arousal threshold and thus, allowing the accumulation of respiratory stimuli to reach a magnitude sufficient to activate the dilator muscles without triggering arousal.13 This treatment involves the administration of a non-myorelaxant hypnotic, such as eszopiclone (eg, Lunesta), which can increase the respiratory arousal threshold by 4 to 5 cm H2O.16 Although studies have found that raising arousal threshold improves AHI scores in some patients, raising the arousal threshold in patients with unresponsive upper airway dilators may be deleterious because hypoxemia could occur prior to arousal from sleep.
With new algorithms, flow data obtained from a standard PSG can be used to directly quantify arousal threshold without a catheter.13,17,18 Alternatively, short respiratory event duration is an indicator of low arousal threshold.
Loop Gain
Loop gain, which is an engineering term, is a value describing the propensity of a system governed by feedback loops to develop unstable behavior. Ventilation is such a system. Loop gain in a ventilation system measures the stability of the negative feedback chemoreflex control system, calculated as the ratio of the ventilatory response to the disturbance that elicited the response (Figure 4).
This tightly controlled, rapidly responsive negative feedback system between the brain and the lungs allows for stable ventilation with relatively small changes in CO2 levels. The oscillating PaCO2 levels in the system stay comfortably within a CO2 reserve of approximately 5 mm Hg.19
During sleep, the levels of CO2 and O2 in the blood dominate ventilatory control. Arterial CO2 has the greater influence, and an increase in CO2 stimulates an increase in ventilatory drive. Ventilatory drive determines not only the level of activity of the thoracic pump muscles but also the upper airway dilator muscles.14 When the CO2 level is low as a consequence of hyperventilation, the neural drive to the upper airway muscles is reduced and the upper airway becomes susceptible to collapse.
A low loop gain (ie, < 1) is consistent with stable breathing. The initial ventilatory response may partially correct the changes in blood gas levels, with any residual changes being gradually corrected later. This is a stable response that is considered to have low loop gain.
High loop gain is a consequence of hypersensitive ventilatory control. This occurs when the initial ventilatory response results in an "overcorrection" or "overcompensation" of the changes in gas levels such that they are better than they were before the perturbation. High loop gain is an indicator of less stable control because a disproportionately large ventilatory response will result in a greater degree of hypocapnia and a subsequent reduction in ventilatory drive. In this manner, a high loop gain (ie, > 1) contributes to the perpetuation of apneas.14
Predictors of OSA resolution with oral appliance therapy are now available because recent studies have revealed that those who responded well had airways that were less likely to collapse (ie, a more negative Pcrit) and lower baseline loop gain when compared with those who responded poorly. Oral appliance therapy improves upper airway collapsibility by addressing anatomical traits; it does not affect muscle dilator effectiveness, loop gain, or arousal thresholds. Those who responded poorly to oral appliance therapy experienced improvement in their upper airway anatomy and collapsibility, but these patients still had residual OSA due to nonanatomic contributions (ie, high loop gain).
In fact, anatomical treatments for sleep apnea may be ineffective in those with excessively high loop gain. Fortunately, measurement of the underlying loop gain can enable clinicians to provide appropriate alternative therapies to these patients because high loop gain is a modifiable factor in one-third of patients with OSA. In addition, loop gain can be used to predict the effectiveness of surgical procedures.20 Supplemental O2 can lower loop gain by 50%, and acetazolamide can lower loop gain by 40% to 50%. The effectiveness of CO2 stabilization will depend on the baseline value. Algorithms can be used to collect loop gain data from the results of a standard PSG.13,21
Muscle Recruitment
The magnitude of the stimuli (ie, from both negative pressure and chemostimulation) required to recruit upper airway dilator muscles adequately to overcome negative intrapharyngeal closing pressures is called the upper airway recruitment threshold (Figure 5). Poor upper airway muscle responsiveness increases the duration of obstructive events because greater stimuli are required to activate the muscles to terminate the obstruction. If the upper airway muscle responsiveness is sufficiently poor, then arousal is necessary to initiate airway opening. The increased chemoreflex drive resulting from both the prolonged obstruction and the accompanying arousal serves to increase the ventilatory response after airway opening. Thus, poor upper airway muscle recruitment interacts with the arousal threshold and loop gain to contribute to repetitive apnea.14 Interestingly, enhanced upper airway muscle responsiveness is a distinct feature among overweight or obese individuals without OSA.22
Improved muscle compensation is associated with a reduction in AHI score. Muscle training can decrease an AHI score by 50%, and hypoglossal nerve stimulation can decrease it by 50% to 70%. Pharmacologic treatments, such as the administration of desipramine, can decrease Pcrit by 2 to 3 cm H2O, significantly improving genioglossus muscle responsiveness and, consequently, upper airway collapsibility in patients with OSA.23
Combining medications such as oxybutynin and atomoxetine boosts responsiveness during non-rapid eye movement sleep. Oxybutynin blocks receptors for acetylcholine on hypoglossal motor neurons, making the genioglossus muscle more responsive during rapid eye movement sleep, and atomoxetine prevents norepinephrine from being resorbed by the neurons that release it, increasing its signal.24
Upper airway muscle responsiveness can be evaluated by using algorithms on data collected from a standard PSG.13,25
Discussion
The causes of SRBDs are multifactorial, not just anatomically driven; thus, assessments of anatomic and nonanatomic contributions should be used to direct trait-targeted therapies. Trait targets include high Pcrit, low arousal threshold, high loop gain, and poor muscle responsiveness. Because each trait-targeted therapy provides a limited benefit, for cases involving more than one trait, combination therapy may be required to achieve a stable airway (Table 1).5
Given the heterogeneity of the causes of SRBDs, it is no wonder that many clinicians have gone down the unscientific path of "trial and error" treatments26 (Figure 6). New diagnostic methods to measure a patient's underlying physiologic traits using routine clinical sleep study data have removed some of the uncertainty and empowered clinicians to deliver individualized therapy for SRBD treatment (Figure 7).14,27,28,29 Maintaining a collaborative mindset among dental, medical, and allied health professionals will lead to evidence-based treatment decisions and optimize patient airway and sleep outcomes.
About the Author
Steven Lamberg, DDS
Diplomate
American Board of Dental Sleep Medicine
Scientific Advisor
Kois Center
Seattle, Washington
Private Practice
Northport, New York
References
1. Benjafield A, Valentine K, Ayas N, et al. Global prevalence of obstructive sleep apnea in adults: estimation using currently available data. American Journal of Respiratory and Critical Care Medicine. 2018;197:A3962.
2. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318.
3. American Dental Association. Proposed policy statement on the role of dentistry in the treatment of sleep-related breathing disorders. American Dental Association Website. http://www.ada.org/~/media/ADA/Member%20Center/FIles/Role_of_Dentistry_in_the_Treatment_of_Sleep_1-5.pdf?la=en. Accessed November 21, 2018.
4. Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13(1):81-88.
5. Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - New pathways for targeted therapy. Sleep Med Rev. 2018;37:45-59.
6. Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79-90.
7. Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985). 2011;110(6):1627-1637.
8. Gleadhill IC, Schwartz AR, Schubert N, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300-1303.
9. Azarbarzin A, Sands SA, Taranto-Montemurro L, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep. 2017;40(1). doi:.1093/sleep/zsw005.
10. Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996-1004.
11. Gold AR, Marcus CL, Dipalo, et al. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121(5):1531-1540.
12. Meurice JC, Marc I, Carrier G, et al. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153(1):255-259.
13. Sands SA, Edwards BA, Terrill PI, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(9):1187-1197.
14. Deacon NL, Jen R, Li Y, et al. Treatment of obstructive sleep apnea. Prospects for personalized combined modality therapy. Ann Am Thorac Soc. 2016;13(1):101-108.
15. Yamauchi M, Fujita Y, Kumamoto M, et al. Nonrapid eye movement-predominant obstructive sleep apnea: detection and mechanism. J Clin Sleep Med. 2015;11(9):987-993.
16. Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011;120(12):505-514.
17. Sands SA, Terrill PI, Edwards BA, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41(1). doi: 10.1093/sleep/zsx183.
18. Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293-1300.
19. Naughton MT. Loop gain in apnea: gaining control or controlling the gain? Am J Respir Crit Care Med. 2010;181(2):103-105.
20. Joosten SA, Leong P, Landry SA, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep. 2017;40(7). doi: 10.1093/sleep/zsx094.
21. Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45(2):408-418.
22. Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190(8):930-937.
23. Taranto-Montemurro L, Sands SA, Edwards BA, et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J. 2016;48(5):1340-1350.
24. Wadman M. Drug pair shows promise for treating sleep apnea. Science. 2018;361(6408):1174-1175.
25. Loewen AH, Ostrowski M, Laprairie J, et al. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34(8):1061-1073.
26. Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for OSA. Chest. 2018;153(3):744-755.
27. Osman AM, Carter SG, Carberry JC, et al. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21-34.
28. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
29. Lim DC, Sutherland K, Cistulli PA, et al. P4 medicine approach to obstructive sleep apnoea. Respirology. 2017;22(5):849-860.