You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The ADAA has an obligation to disseminate knowledge in the field of dentistry. Sponsorship of a continuing education program by the ADAA does not necessarily imply endorsement of a particular philosophy, product, or technique.
For the dental community, the increasing life span of the average person will result in patients who require more dental health care for a longer period of time. As gingival recession is a condition that only worsens with age, its contribution to dental hypersensitivity cannot be ignored. Statistics show that approximately one-third of the nearly 78 million American adults, the baby boomers, over age 60 are at risk for recession in one or more teeth. With the impending flood of dentinal hypersensitivity cases that are likely to result from this demographic, the treatment of dental hypersensitivity is more important than ever. When a patient presents with dental hypersensitivity, dental professionals have an obligation to provide well-considered recommendations for treatment, whether in the office, at home, or both. We will see an increase in the need to provide our patients with recommendations for all levels of dental treatment based upon the changing demographics. This course will discuss factors contributing to and treatment methods for dental sensitivity.
Demographics and Oral Health
Perhaps of most importance demographically, census data shows that in the U.S. alone there were 78.2 million baby boomers in July 2005. This group represents a large aging population with particular oral health needs. The oldest baby boomers turned 60 in 2006. Currently there are over 36 million adults 65 years or older.1 By 2050 this number will grow to over 80 million (Figure 1). This translates into an increase in the number of patients who will need dental services for the treatment of caries and periodontal disease. These same patients will require additional care for pain and trauma, tooth hypersensitivity, and failures of existing dental restorations, along with a greater demand for replacement of missing teeth.
According to a recent report, over 70 percent of adults aged 65 and older currently have some or most of their natural teeth.1 It is expected that the number of edentulous patients will remain stable at nine million adults until 2020.2 Adults currently over age 60 have an average of 19 teeth.3 Over 90 percent of these individuals will have at least one coronal carious lesion. Among this group in 2002, almost 32 percent had root caries or a restored root surface. Since root caries is an indication of recession, this means that at least 30 percent of adults over age 60 are at risk for recession plus root caries in one or more teeth and highlights the risk of root caries in addition to the risk of dentinal hypersensitivity in the presence of gingival recession. Another study concluded that at least 22 percent of the adult population between 30 and 90 years of age will have evidence of recession of 3 mm or greater in one or more teeth.4
Gingival recession is a common condition that increases as we age.5 When gingival recession is present, patients are at risk for pain due to the exposed root surface and root caries, and they may also have aesthetic concerns.5 Clinically, patients are concerned whenever there is dental pain.6 One aspect of routine dental treatment that has been on the rise is the reported presence of dentinal hypersensitivity (Figure 2).
Dentinal hypersensitivity refers to a sharp, sudden pain when teeth are exposed to a stimulus.7 Stimuli can be tactile sensations (such as those that occur during brushing or flossing, or when an explorer is rubbed over the root dentin) or changes in temperature (such as those that occur with hot or cold food, beverages, or even air). No vital tooth in the mouth is immune to dentinal hypersensitivity. Root sensitivity has been reported on incisors, canines, premolars, and molars. The prevalence of dentinal hypersensitivity in the general population has been reported at up to 57 percent.8,9,10,11,12,13,14 Among periodontal patients, the frequency of tooth hypersensitivity is considerably higher (60 percent to 98 percent).15,16
Physiology and Etiology of Dentinal Hypersensitivity
In short review, a tooth consists of four basic tissues; enamel, dentin, cementum, and pulp. Enamel is the hardest tissue in the body and covers the anatomical crown of the tooth. Dentin is found throughout the crown and root of the tooth and surrounds the pulp of the tooth, where the nerves and blood supply is located. Cementum is the calcified cover of the anatomical root of the tooth.
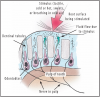
Dentinal hypersensitivity has been a recurring issue in clinical dentistry. In 1884 Calvo wrote: "There is great need of a medicament, which while lessening the sensitivity of dentin, will not impair the vitality of the pulp."17 The most widely and still accepted mechanism of dentinal hypersensitivity was first described by Brännström in 1963.18 According to his hydrodynamic theory, the aspiration of odontoblasts into the dentinal tubules as an immediate effect of physical stimuli applied to exposed dentin results in the outward flow of the tubular contents (dentinal fluids) through capillary action (Figure 2). The changes to the dentinal surface lead to stimulation of the A-type nerve fibers surrounding the odontoblasts. Within the dentinal tubules there are two types of nerve fibers, myelinated (A-fibers) and unmyelinated (C-fibers).19 The A-fibers are responsible for the sensation of dentinal hypersensitivity. Another theory that has been proposed is an alteration in pulpal sensory nerve activity.20
Why are some root surfaces hypersensitive while others are not? Absi and his coworkers reported that nonsensitive teeth were not responsive to any type of physical stimulus and had few exposed dentinal tubules.21 Conversely, sensitive teeth had as many as eight times the number of open dentinal tubules per surface area when compared to the nonresponsive teeth. Dentin is a mineralized connective tissue composed of hydroxyapatite, an inorganic component, and an organic matrix of collagenous proteins. Dentin has different configurations and diameters in different teeth. For human dentin, one square millimeter of dentin can contain 30,000 tubules depending on depth.
With the root surfaces exposed to the oral environment, the dentinal tubules must be opened at both ends, to the pulp and to the oral cavity, for a patient to develop dentinal hypersensitivity (Figure 3). In normal function, the tubules sclerose and become plugged. However, when dentin is cut or abraded, the mineralized matrix produces debris that spreads over the dentin surface to form a smear layer.22,23 This phenomenon occurs to both enamel and dentin, but the loss of this smear layer (the unplugging of the dentinal tubules) contributes to dentinal hypersensitivity.23 When the root surface is exposed, the physical action of brushing with toothpaste can be a predisposing factor in removing the smear layer, leaving a tooth hypersensitive.24 The opening of dentinal tubules can also result from poor oral hygiene techniques, leaving bacterial plaque on root surfaces. The acidic by-products of the plaque can open the dentinal tubules. Also, otherwise excellent oral hygiene techniques but with highly abrasive dentifrices, as in dentifrices to enhance whitening, can cause continued dentinal tubule exposure. Another at-risk occurrence is the exposure of the oral cavity to acids, such as with the consumption of acidic foods and beverages, exposure to chlorinated pool water, or physiological conditions including bulimia and gastroesophageal reflux disease.25,26,27
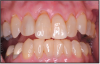
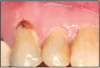
Exposed root surfaces due to gingival recession are the most significant contributor to dentinal root hypersensitivity (Figure 4).27 Common causes of gingival recession include inadequate attached gingiva, prominent roots, toothbrush abrasion, periodontal surgery, oral parafunctional habits (picking at cervical of tooth with fingernail), excessive tooth cleaning, excessive flossing, loss of gingival attachment due to specific pathologies, and iatrogenic loss of attachment during restorative procedures.27 Once the root surfaces are exposed, the cementum/dentin is more susceptible to caries and loss of tooth substance due to erosion, abrasion, and abfraction (Figure 5).28,29,30,31
Root caries progresses more rapidly than coronal caries even in the absence of any predisposing factors such as xerostomia. The organic matrix in cementum comprises a larger proportion of its structure than in dentin and even more so compared to enamel, and the inorganic component is less, making the cementum more susceptible. From a tooth morphology perspective, once gingival recession has occurred and the root is exposed, some roots will have an area with no cementum covering dentin, and for the majority that do have a continuous cementum layer, it is very thin in cross-section compared to the harder and thicker layer of enamel coronally protecting dentin. Furthermore, baby boomers are an at-risk group for xerostomia (drug induced, immuno-suppressed, radiation induced, and other) - further increasing the risk of a combination of dentinal hypersensitivity and root caries.
Treatment of Dentinal Hypersensitivity
Over the years, dentinal hypersensitivity on root surfaces of teeth caused by external stimuli has been treated to control the pain. Before treatment of dentinal hypersensitivity is initiated, a diagnosis must be made. As stated earlier, the diagnosis of dentinal hypersensitivity is one of exclusion. Questioning the patient for the cause of pain and physical and radiographic evaluation of the site(s) for pain must exclude all symptoms that might relate to other causes.6 Once all other causes can be eliminated, the diagnosis of dentinal hypersensitivity can be made. With the correct diagnosis a plan can be developed and implemented for treatment (Table 1).
A definitive diagnosis of dentinal hypersensitivity should lead the dental professional to provide both patient counseling and one or more prescribed treatment courses.
Patient Counseling
Since acidic substances can contribute to the opening of dentinal tubules, a dietary analysis, history of bulimia, dieting, consumption of acidic drinks and foods, or history of gastroesophageal reflux disease (GERD) must be taken into account. Bulimia is recognized by noting the severe wear on the lingual and the most posterior surfaces of teeth due to digestive acids in the vomit. Patient counseling in this area must be approached from medical professionals. A balanced diet focused on the reduction of acid producing foods is important. Certain examples include the reduction of artificial sweeteners, chocolate, peanuts, prunes, milk, and soft drinks. Patients can receive basic dietary information from the dental team as well as more specific information from a registered dietician. GERD is often treated with medication and should be noted in the patient's medical history.
Recommendations to the patient to avoid or minimize acid damage can be made, as acid attacks the tooth surfaces and combined with brushing with toothpaste can lead to further tooth loss and opening of the distal ends of the dentinal tubules as well as increasing the patient's caries risk due to demineralization.
Desensitizing Treatment
Desensitizing treatment of dentin on root surfaces can be accomplished in the office with professional treatments or at home with the use of desensitizing dentifrices. At the time of treatment recommendation, consideration should also be given to the individual patient's caries and risk. It is well accepted that exposed root surfaces are one of the risk factors for future caries. As the baby boomers age, the overall incidence and risk of both sensitivity and root caries will increase. A carefully considered treatment plan should include such evaluation and consider the use of prescription-level fluoride as a caries preventive in combination with desensitizing treatment.
Mechanisms of Action for Desensitizing Agents
The mechanism for desensitizing teeth can be a blockage of nerve response in the pulp, a reduction in dentinal tubule flow, or both. Blockage of nerve activity and the transmission of pain have been reported with the use of potassium nitrate or potassium chloride, both of which have been active ingredients in toothpastes for at-home application. In one clinical trial, the direct application of potassium nitrate solution to hypersensitive dentin demonstrated a reduction in dentinal pain.32 Reduction in tubule fluid flow can be accomplished with surface blockers or agents that create a new smear layer. Over the years, tubule blocking agents have included precipitates on the dentinal surface with potassium and ferric oxalates, aluminum, fluorides, hema (with and without glutaraldehyde), and sealants ranging from restorative materials and dental resins to glass ionomers.33,34,35,36 More recently, lasers have been introduced as an additional in-office professional treatment option.
Professional In-Office Treatment of Dentinal Hypersensitivity
Where in-office treatment is selected either alone or in combination with home therapy, the choice of in-office treatment should consider the following factors: effectiveness, invasiveness, caries risk, loss of tooth structure and tooth contour, patient tolerance/ acceptance, cost, aesthetics, and oral hygiene.
In-Office Operative Treatments
Loss of Tooth Structure and Contour
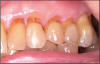
When the exposed, sensitive root surface has surface loss due to abrasion, erosion, and/or abfraction, it leaves a notching appearance of the root. Consideration should be given to using either an adhesive composite resin or glass ionomer restoration.36 These will restore the tooth to full contour and seal the exposed and open dentinal tubules (Figure 6).
Loss of Periodontium Over the Exposed Root Area
Depending on the area of loss and the individual patient's acceptance and desire, periodontal treatment with gingival grafts should be considered as part of a treatment plan.37,38
More recently, lasers have been used successfully to seal open dentinal tubules either by themselves or in concert with surface treatments.39,40,41 The use of an erbium:yttrium-aluminum-garnet (Er:YAG) laser has been shown to be effective for desensitizing hypersensitive dentin for up to six months.39 The desensitizing effect was attributed to the deposition of insoluble salts into the exposed dentinal tubules. Another study investigated the use of an helium-neon (HeNe) laser and a combined HeNe:neodymium- doped yttrium aluminum garnet (Nd:YAG) laser for the treatment of dentinal hypersensitivity.40 This study indicated that both treatments reduced dentinal hypersensitivity by more than 60 percent for up to three months. Surface sealing of patient dentinal tubules using a combined bioglass paste with an neodymium-doped yttrium aluminum perovskite (Nd:YAP) laser has also been demonstrated.41
Another treatment method-iontophoresis-utilizes a low galvanic current to accelerate ionic exchanges and precipitation of insoluble calcium with fluoride gels to occlude open tubules.37
In-Office Surface Treatments
A popular and noninvasive approach to treating root hypersensitivity is the use of in-office paint-on surface treatments. A variety of products has been used to reduce dentinal hypersensitivity, including resin-based materials.34,35 Use of five percent neutral sodium fluoride varnish (Colgate®Duraphat®, Colgate Oral Pharmaceuticals, Inc.) applied to exposed root surfaces has been clinically proven to be an effective treatment of dentinal hypersensitivity for up to six months.42 This product has the benefit of providing a rich source of fluoride to the root surface during desensitization. Other fluoride varnishes have also been used, including Fluor Protector (Ivoclar Vivadent®). DentinBloc®, which blocks tubules using a combination of three fluorides (sodium, stannous, and hydrogen), is an option for rapid, temporary preprocedural relief. An aqueous solution of five percent glutaraldehyde and 35 percent hema (hydroxyethylmethacrylate), Gluma® Desensitizer (Heraeus-Kulzer) has been reported to be an effective desensitizing agent for up to nine months.34,38 The mechanism for tubule occlusion by Gluma Desensitizer appears to be due to the glutaraldehyde effects.43 The use of oxalates for the treatment of dentinal hypersensitivity has been shown to be effective as well.44,45 The oxalate precipitates on the open dentinal tubules, occluding them. A dual-action oxalate desensitizer with potassium nitrate (D/Sense® 2, Centrix) has demonstrated effective desensitizing properties.44 This product combines occlusion of open dentinal tubules with the desensitizing effect of potassium nitrate.
Recently, bioactive glasses containing hydroxycarbonate apatite in a prophylaxis paste (NuCare™, Sunstar Americas) have been introduced that have the capacity to seal dentinal tubule surfaces. The basis for this use comes from a clinical trial using the same active ingredient that is used in toothpastes for the treatment of dentinal hypersensitivity.46
The recommendations for and techniques of use are product-specific. The dental professional needs to understand which in-office desensitizing agents are appropriate to use, giving careful consideration to the patients' risk factors. In some cases, the placement of a restoration may be indicated.
At-Home Treatment of Dentinal Hypersensitivity
Over-the-counter desensitizing dentifrices, usually in the form of toothpastes, are a major category of dentifrice. In the United States, the change in demographics for people over age 50, the fastest- growing segment of our population, will lead to many more people needing and using desensitizing toothpastes.
What are desensitizing toothpastes, and do they work? Dentifrices claiming a desensitization effect come under scrutiny by the U.S. Food and Drug Administration. The claim must be substantiated by either clinical trials or the addition to the toothpaste of an ingredient recognized as being an effective, active agent for the treatment of the condition listed, at the FDA-accepted therapeutic concentration. Because the addition of fluorides to toothpastes has been shown to reduce caries, a claim can be made that the presence of different types of fluoride additives to toothpastes reduces caries.
Two potassium compounds, potassium chloride and potassium nitrate, have been added to toothpastes to reduce dentinal hypersensitivity. While both have been shown to reduce sensitivity, potassium nitrate is the more effective (and popular) of the two.47,48 According to the FDA monograph, for a toothpaste to be desensitizing it must contain 5 percent potassium nitrate as an active ingredient. Potassium nitrate's mode of action has been described as a penetration of the potassium ions through the tubules to the A-fibers of the nerves of the pulp, where repolarization of these fibers is prevented after initial depolarization.49,50,51 The potassium levels act to block the potential for action generated in interdental nerves. If elevated levels of potassium nitrate are maintained, the depolarized state decreases the perception of pain. It can almost be described as a numbing effect on dentinal hypersensitivity.
A second type of desensitizing toothpaste that has been popular for the treatment of dentinal hypersensitivity is a gel that contains 0.4 percent stannous fluoride (Gel-Kam®) and acts by blocking the tubules. A third type is a dentifrice containing 0.4 percent stannous fluoride and 5 percent potassium nitrate for desensitization. This has been found in clinical studies to be an effective desensitizer.44 The currently available popular over-the-counter desensitizing dentifrices contain 1,000 - 1,100 ppm fluoride, which is the same level of fluoride contained in regular fluoride dentifrices and provides the same level of caries protection. Patients who experience dentinal hypersensitivity have varying degrees of risk for root caries due to their exposed root surfaces. While such patients can benefit from additional fluoride protection, it may be unrealistic to expect that many patients will be compliant in the use of more than one dentifrice - one for hypersensitivity and another containing a high, prescription level of fluoride.
More recently, desensitizing dentifrices have become available with prescription-level 5,000 ppm neutral sodium fluoride and potassium nitrate, providing a high level of fluoride for additional caries protection for patients at risk for root caries. For these to be considered effective desensitizers by the FDA, they must contain the FDA-monographed level of five percent potassium nitrate. With a combined five percent potassium nitrate and 5,000 ppm prescription-level fluoride, such dentifrices provide a clinically appropriate desensitizing treatment and provide the best possible level of fluoride protection against root caries on exposed roots with at-home use. The 5,000 ppm fluoride dentifrice Colgate® Prevident® has been clinically proven to remineralize root caries by up to 57 percent over a six-month period.52
Clearly the least invasive treatment option the dental professional has available is the recommendation of a dentifrice product for home use, whether an over-the-counter or prescription dentifrice. Many desensitizing toothpastes contain fluoride for an anticaries effect and may contain antitartar, antigingivitis, and/or whitening ingredients. There is no doubt that toothpastes containing 5 percent potassium nitrate are effective in reducing hypersensitivity of the dentin and root. Many clinical trials have provided evidence of a reduction in tooth sensitivity with potassium nitrate.53,54,55,56,57,58 The patient should be informed that in most cases it takes approximately two weeks of using the dentifrice before any relief is noticed. Continued use is required to avoid relapse.
Summary
As patients age, they will naturally experience gingival recession and become more susceptible to dental hypersensitivity. The diagnosis of dentinal hypersensitivity is one of exclusion. One of the questions that dental professionals should ask patients during every recare/recall appointment is whether they are experiencing sensitivity with any teeth. Based upon evaluation and the discovery that dentinal hypersensitivity is the diagnosis, the dental professional can make recommendations for treatment. For individual teeth that are hypersensitive, an in-office treatment can be applied to provide the patient with pain relief. Once teeth are predisposed to dentinal hypersensitivity, patients will need to be evaluated for at-home treatment. The least invasive treatment is a desensitizing toothpaste. The combination of five percent potassium nitrate and 1.1 percent neutral sodium fluoride (5,000 ppm fluoride) provides clinicians with the possibility of providing ongoing treatment for desensitization and a high-level fluoride toothpaste for at-home preventive care of exposed root surfaces. Consideration needs to be given to the patient's status, including caries rate and hypersensitivity experience and risk, as the dental professional recommends treatment.
Glossary
Abfraction - loss of tooth structure from flexural forces.
Baby Boomers - babies born during the post WW II era from the approximate years of 1945-1964.
Bulimia - eating disorder characterized by binge eating and purging.
Collagen- a group of naturally occurring proteins. It is the most abundant protein in mammals.
Demineralization - excessive elimination of mineral or organic salts from tissues of the body.
Dentinal tubules - microscopic canals found in the dentin.
Etiology - study of the causes of disease.
Iatrogenic disease - condition caused by a doctor's statements or procedure.
Hypersensitivity- state of being excessively sensitive to a substance.
Morphology - the science of form and structure.
Myelinated - having a myelin sheath: myelinated nerve fibers.
Odontoblasts - one of the cells forming the outer surface of dental pulp that produce the dentin of a tooth.
Periodontium - specialized tissues that both surround and support the teeth.
Physiology- science which deals with the functions of the body.
Precipitate- a formation of a solid during a chemical reaction.
Recession - the process of withdrawal or wearing away from the normal location.
Repolarization - the return of cell membrane potential to resting.
Sclerose - to become or cause to become hardened or sclerotic.
Tactile - pertaining to sense of touch.
Xerostomia - subjective complaint of dry mouth due to lack of saliva.
References
1. Available at: https://www.census.gov/population/projections/natsum-T3.html
(accessed January 8, 2006.)
2. Ettinger RL. The unique oral health needs of an aging population. Dent Clin North Am 1997;41(4):633-49.
3. Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, Hyman J, Jaramillo F, Kingman A, Nowjack- Raymer R, Selwitz RH, Wu T; Centers for Disease Control and Prevention (CDC). Surveillance for dental caries, dental sealants, tooth retention, edentulismand enamel fluorosis- United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 2005; 54(3):1-43.
4. Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R, et al. Gingival recession, gingival bleeding and dental calculus in adults 30 years of age and older in the United States, 1988- 1994. J Periodontol 1999; 70:30-43.
5. Tugnait, Clerehugh V. Gingival recession-its significance and management. J Dent 2001; 29:381-94.
6. Strassler HE, Gerhardt DE. Trouble shooting everyday restorative emergencies. Dent Clin North Am. 1993 37(3):353- 65.
7. Holland GR, Narhi MN, Addy M, Gangarosa, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol 1997; 24:808-13.
8. Rees JS. The prevalence of dentine hypersensitivity in general dental in the UK, J Clin Periodontal 2000; 27:860-5.
9. Irwin CR, McCusker P. Prevalence of dentine hypersensitivity in a general dental population. J Ir Dent Assoc 1997; 43:7-9.
10. Clayton DR, McCarthy D, Gillam DG. A study of the prevalence and distribution of dentine sensitivity in a population of 17-58-year-old serving on an RAF base in the Midlands. J Oral Rehabil 2002; 29:14-23.
11. Al-Sabbagh M, Andre3anna S, Ciancio SG. Dentinal hypersensitivity: review of aetiology, differential diagnosis, prevalence and mechanism. J Int Acad Periodontol 2004; 6(1):8-12.
12. Fischer C, Fischer RG, Wennberg A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio de Janeiro, Brazil. J Dent 1992; 20:272-76.
13. Liu HC, Lan WH, Hsieh CC. Prevalence and distribution of cervical dentin hypersensitivity in a population in Taipei, Taiwan. J Endod 1998; 24:45-47.
14. Taani DQ, Awartani F. Prevalence and distribution of dentin hypersensitivity and plaque in a dental hospital population. Quintessence Int 2001; 32:372-76.
15. Chabanski MB, Gillam DG, Bulman JS, Newman HN. Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist Periodontology department. J Clin Periodontol 1996; 23:989-92.
16. von Troil B, Needleman E, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol 2002; 29(Suppl) 3: 173-77.
17. Calvo P. Treatment of sensitive dentine. Dent Cosmos 1884; 139-141.
18. Brännström M. Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc 1963; 66:366-370.
19. Johnson DC. Innervation of teeth: qualitative, quantitative, and developmental assessment. J Dent Res 1985; 64(Spec Issue):555-563.
20. Kim S. Hypersensitive teeth: desensitization of pulpal nerves. J Endod 1986; 12:482-485.
21. Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non- sensitive cervical dentine. J Clin Periodontol 1987; 14(5):280- 284.
22. Eick JD, Wilko RA, Anderson CH, Sorensen SE. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of electron microprobe. J Dent Res 1970; 49:1359-68.
23. Pashley DH. Smear layer: physiological considerations. Oper Dent Suppl 1984; 3:13-29.
24. Addy M. Dentine hypersensitivity: definition, prevalence, distribution and aetiology. In: Addy M. Embery G, Edgar WM. Orchardson R editors. Tooth wear and sensitivity. Clinical advances in restorative dentistry. London Martin Dunitz; 2000; p. 239-48.
25. Corrêa FOB, Sampaio JEC, Júnior CR, Orrico SRP. Influence of natural fruit juices in removing the smear layer from root surfaces-an in vitro study. J Can Dent Assoc 2004; 70:697- 702.
26. Rees JS, Loyn T, Rowe W, Kunst Q, McAndrew R. The ability of fruit teas to remove the smear layer: an in vitro study of tubule patency. J Dent. 2005; 34:67-76.
27. Jacobsen PL, Bruce G. Clinical dental hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract 2001; 2(1):1-8.
28. Piotrowski BT, Gillette WB, Hancock EB. Examining the prevalence and characteristics of abfractionlike cervical lesions in a population of U.S. veterans. J Am Dent Assoc 2001; 132:1694-1701.
29. Braem M, Lambrechts P, Vanderle G. Stress induced cervical lesions. J Prosthet Dent 1992; 67:718-22.
30. Smith BG, Knight JK. A comparison of patterns of tooth wear with the etiologic factors. Br Dent J 1984; 157:16-19.
31. Grippo JO. Abfraction: a new classification of hard tissue lesions of teeth. J Esthet Dent 1991; 3:14-19.
32. Touyz LZG, Stern J. Hypersensitive dentinal pain attenuation with potassium nitrate. Gen Dent 1999; 47:42-45.
33. Paes Leme AF, dos Santos JC, Giannini M, Wada RS. Occlusion of dentin tubules by desensitizing agents. Am J Dent 2004; 17:368-72.
34. Duran I, Sengun A. The long-term effectiveness of five current desensitizing products on cervical dentine sensitivity. J Oral Rehabil 2004; 31:351-56.
35. Dondi dall'Orologio G, Lorenzi R, Anselmi M, Opisso V. Dentin desensitizing effects of Gluma Alternative, Health-Dent Desensitizer, and Scotchbond Multi-Purpose. Am J Dent 1999; 12:103-106.
36. Starr GB. Class 5 restorations. In Summitt JB, Robbins JW, Schwartz RS editors Fundamentals of Operative Dentistry a Contemporary Approach 2nd edition. Quintessence Books, Chicago. p. 386-400.
37. Gangarosa L Sr. Iontophoretic application of fluoride in tray techniques for desensitizing multiple teeth. J Am Dent Assoc 1981; 95:50-52.
38. Kakaboura A, Rahiotis C, Thomaidis S, Doukoudakis S. Clinical effectiveness of two agent on the treatment of tooth cervical hypersensitivity. Am J Dent 2005; 18:291-95.
39. Schwarz F, Arweiler N, Georg T, Reich E. Desensitizing effects of an Er:YAG laser on hypersensitive dentine. J Clin Periodontol 2002; 29:211-15.
40. Gelskey SC, White JM, Pruthi VK. The effectiveness of the Nd:YAG laser in the treatment of dentin hypersensitivity. J Can Dent Assoc 1993; 59:377-86.
41. Lee B, Chang C, Chen W, Lan W, et al. In vitro study of dentin hypersensitivity treated by Nd:YAP laser and bioglass. Dent Mater 21:511-19.
42. Gaffar A. Treating hypersensitivity with fluoride varnishes. Compend Contin Educ Dent 1998; 19:1088-1097.
43. Schüpback P, Lutz F, Finger WJ. Closing of dentinal tubules by Gluma desensitizer. Eur J Oral Sci 1997; 105:414-421.
44. Crispin BJ. Dentin sensitivity and the clinical evaluation of a unique dual-action dentin desensitizer. Contemp Esthet Restor Pract (Suppl) 2001; 8(3):3-7.
45. Camps J, Pashley D. In vivo sensitivity of human root dentin to air blast and scratching. J Periodontol 2003; 74:1589-94.
46. Gillam DG, Tang JY, Mordan NJ, Newman HN. The effects of a novel Bioglass dentifrice on dentine sensitivity: a scanning electron microscopy investigation. J Oral Rehabil 2002; 29:305-313.
47. Silverman G, Gingold J, Curro FA. Desensitizing effect of a potassium chloride dentifrice. Am J Dent 1994; 7:9-12.
48. Sowinski J, Ayad F, Petrone M, DeVizio W, et al. Comparative investigations of the desensitizing efficacy of a new dentifrice. J Clin Periodontol 2001; 28:1032-36.
49. Markowitz K, Bilotto G, Kim S. Decreasing intradental nerve activity in the cat with potassium and divalent cations. Archives of Oral Biology 1991; 36:1-7.
50. Peacock JM, Orchardson R. Effects of potassium ions on action potential conduction in A- and C- fibers of rat spinal nerves. J Dent Res 1995; 74:634-641.
51. Markowitz K, Kim S. The role of selected cations in the desensitization of intradental nerves. Proc Finn Dent Soc 1992; 88(Suppl) 1:39-54.
52. Baysan, A et al. Reversal of primary root caries using dentifrices containing 5,000 ppm and 1,100 ppm fluoride. Caries Res. 2001; 35: 41-46.
53. Wara-aswapati N, Krongnawakul D, Jiraviboon D, Adulyanon S, et al. The effect of a new toothpaste containing potassium nitrate and triclosan on gingival health, plaque formation and dentine hypersensitivity. J Clin Periodontol 2005; 32:53-58.
54. Sowinski JA, Battista GW, Petrone ME, Chaknis P, et al. A new desensitizing dentifrice-an 8-week clinical investigation. Compend Contin Educ Dent Suppl 2000; 27:11-16.
55. Schiff T, Bonta Y, Proskin HM, DeVizio W, et al. Desensitizing efficacy of a new dentifrice containing 5.0% potassium nitrate and 0.454% stannous fluoride. Am J Dent 2000; 13:111-15.
56. Sowinski JA, Bonta Y, Battista GW, Ptrone D, et al. Desensitizing efficacy of Colgate Sensitive Maximum Strength and Fresh Mint Sensodyne dentifrices. Am J Dent 2000; 13:116-20.
57. Conforti N, Battista GW, Petrone DM, Petrone ME, et al. Comparative investigaton of the desensitizing efficacy of a new dentrifice : a 14-day clinical trial. Compend Contin Educ Dent Suppl 2000; 27:17-22.
58. Schiff T, Zhang YP, DeVizio W, Stewart B, et al. A randomized clinical trial of the desensitizing efficacy of three dentifrices. Compend Contin Educ Suppl 2000; 27:4-10.
About the Author
Howard E. Strassler, DMD, FADM, FAGD, FACD
Dr. Howard Strassler is professor and director of operative dentistry at the University of Maryland Dental School in the Departments of Endodontics, Prosthodontics, and Operative Dentistry. He has lectured nationally and internationally on techniques and a selection of dental materials in clinical use and aesthetic restorative dentistry. He is a fellow in the Academy of Dental Materials and the Academy of General Dentistry, a member of the American Dental Association, the Academy of Operative Dentistry, and the International Association of Dental Research. He is on the editorial board of numerous publications. He is a consultant and clinical evaluator to more than 15 dental manufacturers. Dr. Strassler has published more than 400 articles in the field of restorative dentistry and innovations in dental practice, and he has coauthored seven chapters in texts. He has presented more than 425 programs, including most of the major programs throughout the United States, Canada, and Europe. Dr. Strassler has a general practice in Baltimore, Maryland, that is limited to restorative dentistry and aesthetics.