You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
At least several times a week, a clinician is likely to encounter patients with sensitive teeth. The phenomenon is typically due to thermal changes in the mouth that occur during drinking or ingesting cold beverages or foods or when inhaling cold air on a winter day. Some patients with hypersensitivity have concerns that the pain indicates a potential toothache or the need for immediate dental attention.
Tooth sensitivity can be due to various reasons, which could include postoperative sensitivity after restorative treatment, a failing restoration that has marginal leakage so that the tooth feels sensitive during normal eating or breathing, or changes in the osmotic gradient due to the types of foods or beverages being ingested. A patient may find it difficult to know the cause of the pain. This article will focus specifically on dentin hypersensitivity.
Patients may report dentin hypersensitivity as a sharp, brief tooth pain irrespective of stimulus.1 Although dentin hypersensitivity can be present in anyone regardless of age, adults are more likely to articulate this complaint. In recent years, baby boomers, individuals who were born between 1946 and 1964, have been aging. In 2014 the youngest baby boomer is 50 years old with the oldest being 68. This portion of the population now nearly numbers 80 million and includes those born in the United States and abroad. More than 40 million adults are 65 years or older.6 By 2050, this will expand to more than 80 million. Also, more than 70% of adults 65 years and older have some or most of their natural teeth. The number of edentulous patients is expected to remain at 9 million until 2020.7 These data strongly suggest a possible increase in the number of patients who will need dental services for the treatment of caries and periodontal disease. These same patients will also likely require more care for dental emergencies due to infection, pain and trauma, tooth hypersensitivity, and failures of existing dental restorations.
Definition
Also known as tooth sensitivity and root sensitivity, dentin hypersensitivity is a common complaint from patients. Holland and colleagues2 described dentin hypersensitivity as “short, sharp pain arising from exposed dentin in response to stimuli typically thermal, evaporative (air), tactile (rubbing), osmotic or chemical and which cannot be ascribed to any other form of dental defect of pathology.” Dental professionals see dentin hypersensitivity as an exaggerated response to routine stimuli imposed on teeth. With a dental examination, radiographs (if necessary), and diagnostic testing, the finding in most cases is dentin hypersensitivity,3,4 which is typically associated with recession. Tooth whitening with peroxide compounds, a routine restorative procedure, appears to cause similar sensitivity. Although the phenomena of both types of tooth sensitivity appear to be the same, their etiologies are different.5 These differences will be presented later in this article.
Dentin hypersensitivity may make patients uncomfortable during periodontal cleanings and may cause pain when toothbrushing or flossing. Although dentin hypersensitivity does not directly harm the tooth, dentin, or pulp, it can be considered a true pain syndrome.8 Sensitivity pain, which is of short duration, should be distinguished from pain of longer duration not treatable with desensitizing agents, which may be the result of pulpal inflammation.9 Typically, dentin hypersensitivity occurs when a tooth is exposed to cold foods, sweets, beverages, or plaque accumulation on exposed root surfaces. Dentin hypersensitivity is considered to be one of the most painful and least successfully treated chronic dental conditions.10
After diagnosis and the etiology has been established, treatment recommendations may include both in-office professionally applied treatments and at-home professionally dispensed therapies or recommendations for over-the-counter treatments.11 Clinical follow-up with the patient is necessary to ascertain the therapeutic benefits of treatment recommendations.
Several studies examining the prevalence of dentin hypersensitivity have reported a rate between 4% and 57% in the general population.12-18 The rate is considerably higher among periodontal patients (60% to 98%).19,20 Patients who have dentin hypersensitivity may not visit the dentist for the complaint because they do not view it as a significant dental health problem but may mention it at a routine dental appointment.21 Generally, dentin hypersensitivity occurs in patients between the ages 30 and 40 years;22 however, patients can be significantly younger or older. Both sexes are affected, although dentin hypersensitivity may be more common in women.23 Root sensitivity has been reported in incisors, canines, premolars, and molars, although it has been observed to be more common in canines and premolars.24,25
Etiology and Physiology
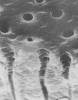
The anatomy and physiology of dentin account for its sensitivity. In 1884, Calvo26 wrote, “There is great need of a medicament, which while lessening the sensitivity of dentin, will not impair the vitality of the pulp.” Dentin is a porous, mineralized connective tissue with an organic matrix of collagenous proteins and an inorganic component. This is known as hydroxyapaptite. Within dentin are microscopic structures known as dentinal tubules, functioning as micro-canals channeling through the dentin from the pulp cavity to the surface cementum border (Figure 1). The dentinal tubules vary in their configurations and diameters. Depending on depth, 1 square millimeter of dentin can contain 30,000 tubules. Each tubule contains a Tomes fiber (cytoplastic cell process) and an odontoblast, which communicates with the pulp. Two types of nerve fibers are within the dentinal tubules: myelinated (Type A fibers) and unmyelinated (Type C fibers).27 Type A fibers cause the sensation of dentin hypersensitivity.
The hydrodynamic theory of dentin hypersensitivity, which was first described by Brännström, partly focuses on dentinal tubules.28,29 When a trigger is present on exposed dentin, odontoblasts aspirate into dentinal tubules. An outward flow of the tubular contents, or dentinal fluids, results through capillary action. These changes lead to stimulation of Type A fibers surrounding the odontoblasts. Pain results when dentinal fluid expansion, contraction, or flow within the dentinal tubules due to thermal changes, evaporative stimuli, or osmotic changes stimulates the pressure-sensitive Type A fibers.28,29 For a stimulus response to occur, the tubules must be open at both ends in the dentinal interface and within the pulp. Kim30 proposed a different theory—an alteration in pulpal sensory nerve activity.
Absi and colleagues31 reported non-sensitive teeth did not show reactions to triggers and had few exposed dentinal tubules. Sensitive teeth had as many as eight times the quantity of open dentinal tubules per surface area when compared with non-sensitive teeth.
Identifying Patients at Risk
Patients are concerned when dental pain occurs32 and can pinpoint areas of dentin hypersensitivity before a clinical examination is performed. If dentin hypersensitivity is identified when blowing air on a tooth or by scratching during a tactile examination of hard tooth surfaces but the patient has not complained of tooth sensitivity, treatment is not indicated. Treatment should be rendered after patient request and examination.
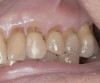
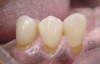
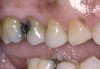
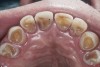
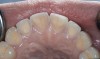
A major predisposing factor to dentin hypersensitivity is exposed root surfaces (Figure 2 through Figure 4).33 Contributors to dentin hypersensitivity include enamel loss with exposed dentin due to attrition and tooth wear caused by bruxism, occlusal habits, and parafunctional activity (Figure 5).34
A study35 examining adults older than 60 years found that almost 32% had root caries or a restored root surface. Root caries can indicate periodontal attachment loss and subsequent recession, suggesting at least 30% of adults older than 60 could be at risk for recession in at least one tooth. Another report36 indicated that at least 22% of adults between 30 and 90 years will exhibit recession of at least 3 mm in one or more teeth. Age increases the rate of gingival recession.37 Patients who have had or are receiving periodontal therapy are also at risk for dentin hypersensitivity.38 Root sensitivity prevalence has been reported to be 9% to 23% before periodontal therapy and 54% to 55% after treatment (scaling and root planing and periodontal surgery). Root sensitivity intensified 1 to 3 weeks following therapy, and then slowly decreased.
Among the causes of gingival recession are inadequate attached gingiva, prominent roots with a thin alveolar housing or bony dehiscence, toothbrush abrasion, excessive tooth cleaning, excessive flossing, periodontal surgery, factitial habits (eg, picking at tooth cervical with fingernail), gingival attachment loss due to specific pathologies, and iatogenic loss of attachment during restorative procedures.39 Upon exposure of the root surfaces, erosion, abrasion, and abfraction make the cementum/dentin more vulnerable to caries and loss of tooth substance.40-43
In dentin hypersensitivity, gingival recession is a predisposing factor, not a direct cause.23 To have the condition, the patient must have dentin tubules open at both ends, to the pulp and oral cavity. For normal conditions, the tubules harden and become plugged. However, when dentin is cut or abraded, the mineralized matrix creates debris that spreads over the dentin surface. A smear layer is then formed.44,45 This phenomenon occurs to both enamel and dentin.45 But the loss of this smear layer, or the unplugging of the dentinal tubules, contributes to dentin hypersensitivity. Root surfaces exposed to toothbrushing with and without the use of toothpaste can be predisposing factors in removing the smear layer.22,46 The opening of dentinal tubules can also happen when the patient does not remove all bacterial plaque from root surfaces. The acidic byproducts of the plaque can open dentinal tubules. The use of highly abrasive toothpaste can lead to continued dentinal tubule exposure. Another patient behavior that presents risk is the exposure of the oral cavity to acids, eg, ingestion of acidic foods and beverages,47-49 ingestion of chlorinated pool water,44 and bulimia and gastrointestinal reflux disease. These risk factors can also contribute to the opening of the end of the dentinal tubules (Figure 6).51 Patient should be advised to refrain from toothbrushing immediately after ingestion of acidic foods or beverages.23
Screening and Diagnosis
Screening for dentin hypersensitivity is not a part of routine examination unless the patient mentions the pain.52 In 1995, Dutch general practitioners were randomly surveyed on the prevalence, conditions, and treatment of cervical hypersensitivity in their practices.53 UK dentists participated in a similar study in 2002.54 The findings from these studies showed that dentists reported 10% of patients having moderate pain and 1% experiencing severe pain from the condition. These results indicated to the researchers that issues in screening, perception, and knowledge of dentin hypersensitivity treatment persist.
In a 2003 questionnaire taken by Canadian dentists and dental hygienists designed to evaluate a practitioner’s perception and clinical management of dentin hypersensitivity,52 fewer than half of the respondents considered differential diagnoses, even though dentin hypersensitivity is a diagnosis of exclusion. A total of 64% of the dentists and 77% of the hygienists identified bruxism and malocclusion as triggers of dentin hypersensitivity. The researchers oberved only 7% of dentists and 5% of dental hygienists correctly chose erosion as a primary cause, and 17% of dentists and 48% of hygienists were not able to pinpoint the commonly accepted hypersensitivity theory.
In this same survey, dentin hypersensitivity management was studied. Only half of the respondents reported they could manage dentin hypersensitivity pain, with only 50% considering the changing predisposing factors as a means to manage pain. The findings showed practitioners may not understand the mechanisms for how desensitizing toothpastes work to relieve pain. More than half of dentists (56%) and dental hygienists (68%) believed desensitizing toothpastes helped prevent dentin hypersensitivity while 31% of dentists and 16% of hygienists did not think desensitizing toothpastes relieved dentin hypersensitivity.
Improved education on the etiology and treatment of dentin hypersensitivity may benefit dental professionals. As part of any screening for dentin hypersensitivity, the clinician should determine whether a localized or generalized problem exists. In-office treatments are usually sufficient for localized isolated tooth dentin hypersensitivity. For generalized conditions in which significant recession is evident on multiple teeth, an at-home treatment regimen may be more suitable.
Tooth Whitening-Induced Sensitivity
Although dentin hypersensitivity and tooth whitening sensitivity appear to be the same symptomatically, their etiologies show that they are not. Patients report dentin hypersensitivity as sharp, short-lasting tooth pain irrespective of the stimulus. Tooth sensitivity caused by tooth whitening has been described as mild to intolerable pain.55-57 Tooth bleaching sensitivity starts during the bleaching procedure but sensitivity levels return to normal after or during the whitening treatment.5 Sensitivity has been the highest-reported adverse reaction in tooth whitening.5,58-60
In clinical research studies, tooth sensitivity during whitening either with at-home tray delivery and in-office procedures has been reported in 18% to 78% of patients.58-60 Clinical observations suggest it is fleeting and has no long-term effects.61 Some dental professionals had believed this transient sensitivity was caused by gingival recession. However, research has shown that gingival recession is not a factor in tooth hypersensitivity when bleaching.62
It has been suggested that the etiology of tooth hypersensitivity during peroxide-based tooth whitening is directly related to the rapid penetration of the peroxide through the enamel and dentin into the pulp, causing a reversible pulpitis.63,64 A correlation has been made that higher concentrations of peroxide-based whitening products have more reports of tooth sensitivity.65,66
For patients at risk, in many cases due to past history of tooth sensitivity while whitening, recommendations to minimize tooth sensitivity during peroxide-based whitening include using lower concentrations of whitening products, using carbamide peroxide bleaching gels, recommending the use of potassium nitrate- and fluoride-containing bleaching gels, and using a potassium nitrate-containing over-the-counter toothpaste applied topically daily for 2 weeks before initiating whitening procedures.5
Conclusion
Dental professionals should understand the causes of hypersensitivity. A patient should be evaluated based on risk factors that may be present. Once a diagnosis has been made and the factors identified, the dental professional can create a treatment plan for the patient’s dentin hypersensitivity. As part of the routine dental examination, dental professionals should ask patients about any sensitive teeth. Depending on severity of the condition, clinical management of dentin hypersensitivity may include both in-office and self-applied at-home therapies.3,67,68
References
1. Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity - an enigma? A review of terminology, mechanisms, aetiology and management. Br Dent J. 1999;
187(11):606-611.
2. Holland GR, Narhi MN, Addy M, et al. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24(11):
808-813.
3. Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51(3):323-332.
4. Chu CH, Lam A, Lo EC. Dentin hypersensitivity and its management. Gen Dent. 2011;59(2):115-122.
5. Hewlett ER. Etiology and management of whitening-induced tooth hypersensitivity. J Calif Dent Assoc. 2007;35(7):499-506.
6. United States Census National Population Projections 2012 Summary Tables. Uinted States Census Bureau website. www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed December 20, 2013.
7. Ettinger RL. The unique oral health needs of an aging population. Dent Clin North Am. 1997;41(4):633-649.
8. Curro FA. Tooth hypersensitivity in spectrum of pain. Dent Clin North Am. 1990;34(3):429-437.
9. Camps J, Pashley D. In vivo sensitivity of human root dentin to air blast and scratching. J Periodontol. 2003;74(11):1589-1594.
10. Silverman G, Berman E, Hanna CB, et al. Assessing the efficacy of three dentifrices in the treatment of dentinal hypersensitivity. J Am Dent Assoc. 1996;127(2):191-201.
11. Orchardson R, Gillam GC. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137(7):990-998.
12. Rees JS. The prevalence of dentine hypersensitivity in general dental practice in the UK. J Clin Periodontol. 2000;27(11):860-865.
13. Irwin CR, McCusker P. Prevalence of dentine hypersensitivity in a general dental population. J Ir Dent Assoc. 1997;43(1):7-9.
14. Clayton DR, McCarthy D, Gillam DG. A study of the prevalence and distribution of dentine sensitivity in a population of 17-58-year-old serving on an RAF base in the Midlands. J Oral Rehabil. 2002;29(1):14-23.
15. Al-Sabbagh M, Andreana S, Ciancio SG. Dentinal hypersensitivity: review of aetiology, differential diagnosis, prevalence and mechanism. J Int Acad Periodontol. 2004;6(1):8-12.
16. Fischer C, Fischer RG, Wennberg A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio de Janeiro, Brazil. J Dent. 1992;20(5):272-276.
17. Liu HC, Lan WH, Hsieh CC. Prevalence and distribution of cervical dentin hypersensitivity in a population in Taipei, Taiwan. J Endod. 1998;24(1):45-47.
18. Taani DQ, Awartani F. Prevalence and distribution of dentin hypersensitivity and plaque in a dental hospital population. Quintessence Int. 2001;32(5):372-376.
19. Chabanski MB, Gillam DG, Bulman JS, Newman HN. Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department. J Clin Periodontol. 1996;23(11):989-992.
20. von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Suppl 3):173-177.
21. Gillam DG, Seo HS, Bulman JS, Newman HN. Perceptions of dentine hypersensitivity in a general practice population. J Oral Rehabil. 1999;26(9):
710-714.
22. Addy M. Dentine hypersensitivity: definition, prevalence, distribution and aetiology. In Addy M, Embery G, Edgar WM, Orchardson R, eds. Tooth wear and sensitivity: Clinical advances in restorative dentistry. London Martin Dunitz; 2000:239-248.
23. Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:375-376.
24. Orchardson R, Collins WJ. Clinical features of hypersensitive teeth. Br Dent J. 1987;162(7):253-256.
25. Addy M, Mostafa P, Newcombe RG. Dentine hypersensitivity: the distribution of recession, sensitivity and plaque. J Dent. 1987;15(6):242-248.
26. Calvo P. Treatment of sensitive dentine. Dent Cosmos 1884;139-141.
27. Johnson DC. Innervation of teeth: qualitative, quantitative, and developmental assessment. J Dent Res. 1985;64(Spec Issue):555-563.
28. Brännström M. Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc. 1963;66:366-370.
29. Brännström M, Aström A. The hydrodynamics of the dentine; its possible relationship to dentinal pain. Int Dent J. 1972;22(2):219-227.
30. Kim S. Hypersensitive teeth: desensitization of pulpal nerves. J Endod. 1986;12(10):482-485.
31. Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14(5):280-284.
32. Strassler HE, Gerhardt DE. Trouble shooting everyday restorative emergencies. Dent Clin North Am. 1993;37(3):353-365.
33. Jacobsen PL, Bruce G. Clinical dental hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2(1):1-12.
34. Smith GBN, Knight JK. A comparison of patterns of tooth wear with the etiological factors. Br Dent J. 1984;157(1):16-19.
35. Beltran-Aguilar ED, Barker LK, Canto MT, et al. Surveillance for dental caries, dental sealants, tooth retention, edentulism and enamel fluorosis-United States, 1988-1994 and 1999-2002. MMWR Surveill Summ. 2005;54(3):1-43.
36. Albandar JM, Kingman A. Gingival recession, gingival bleeding and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70(1):30-43.
37. Tugnait A, Clerehugh V. Gingival recession-its significance and management. J Dent. 2001;29(6):
381-394.
38. von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Supplement 3):173-177.
39. Jacobsen PL, Bruce G. Clinical dental hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2(1):1-12.
40. Piotrowski BT, Gillette WB, Hancock EB. Examining the prevalence and characteristics of abfractionlike cervical lesions in a population of US veterans. J Am Dent Assoc. 2001;132(12):1694-1701.
41. Braem M, Lambrechts P, Vanderle G. Stress-induced cervical lesions. J Prosthet Dent. 1992;67(5):
718-722.
42. Smith BG, Knight JK. A comparison of patterns of tooth wear with the aetiological factors. Br Dent J. 1984;157(1):16-19.
43. Grippo JO. Abfractions: a new classification of hard tissue lesions of teeth. J Esthet Dent. 1991;3(1):14-19.
44. Eick JD, Wilko RA, Anderson CH, Sorensen SE. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of electron microprobe. J Dent Res. 1970;49(6):1359-1368.
45. Pashley DH. Smear layer: biologic considerations. Oper Dent Suppl. 1984;3:13-29.
46. Addy M. Tooth brushing, tooth wear, and dentine sensitivity-are they associated? Int Dent J. 2005;55(4 Suppl 1):261-267.
47. Corrêa FOB, Sampaio JEC, Rossa Júnior C, Orrico SR. Influence of natural fruit juices in removing the smear layer from root surfaces-an in vitro study. J Can Dent Assoc. 2004;70(10):697-702.
48. Rees JS, Loyn T, Rowe W, et al. The ability of fruit teas to remove the smear layer: an in vitro study of tubule patency. J Dent. 2005;34(1):67-76.
49. Sauro S, Mannocci F, Watson TF, et al. The influence of soft acidic drinks in exposing dentinal tubules after non-surgical periodontal treatment: a SEM investigation on the protective effects of oxalate-containing phytocomplex. Med Oral Patol Oral Cir Bucal. 2007;12(7):E542-E548.
50. Geurtsen W. Rapid general dental erosion by gas-chlorinated swimming pool water. Review of the literature and case report. Am J Dent. 2000;13(6):291-293.
51. Carlaio RG, Grassi RF, Losacco T, et al. Gastroesophageal reflux disease and dental erosion. A case report and review of the literature. Clin Ter. 2007;
158(4):349-353.
52. Canadian Advisory Board on Dentin Hyper-sensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69(4):221-226.
53. Gillam DG, Bulman JS, Eijkman MA, Newman HN. Dentists’ perceptions of dentine hypersensitivity and knowledge of its treatment. J Oral Rehabil. 2002;29(3):219-225.
54. Fernandez PM, Rosenblum M, Markowitz K, Zhu H. Relationship between observable dentinal tubules to thermal sensitivity of dentin. J Dent Res. (Special Issue A). 2004;83:Abstract 1675.
55. Sterrett J, Price RB, Bankey T. Effects of home bleaching on the tissues of the oral cavity. J Can Dent Assoc. 1995;61(5):412-420.
56. Tam L. Effect of potassium nitrate and fluoride on carbamide peroxide bleaching. Quintessence Int. 2001;
32(10):66-70.
57. Haywood VB. Current status of nightguard vital bleaching. Compend Contin Educ Dent. 2000;21(Suppl 28):S10-S17.
58. Haywood VB, Cordero R, Wright K, et al. Brushing with a potassium nitrate dentifrice to reduce bleaching sensitivity. J Clin Dent. 2005;16(1):17-22.
59. Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200
(7):371-376.
60. Jorgensen MG, Carroll WB. Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc. 2002;133(8):1076-1082.
61. Swift EJ Jr. At-home bleaching: pulpal effects and tooth sensitivity issues, part II. J Esthet Restor Dent. 2006;
18(5):301-305.
62. Gerlack RW, Barker ML, Anastasia MK, et al. Gingival recession and clinical response with extended whitening strip use. J Dent Res. 2005;84(Special Issue A):Abstract 2124.
63. Haywood VB. Treating tooth sensitivity during whitening. Compend Contin Educ Dent. 2005;26(suppl 3):11-20.
64. Cooper JS, Bokmeyer TJ, Bowles WH. Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod. 1992;18(7):315-317.
65. Matis BA, Mousa HN, Cochran M, Eckert GJ. Clinical evaluation of bleaching agents of different concentrations. Quintessence Int. 2000;31(5):303-310.
66. Kihn PW, Barnes DM, Romberg E, Peterson K. A clinical evaluation of 10 percent vs. 15 percent carbamide peroxide tooth-whitening agents. J Am Dent Assoc. 2000;131(10):1478-1484.
67. Strassler HE, Serio FG. Dentinal hypersensitivity: etiology, diagnosis, and management. Dental Economics. 2009;99(12)(CE Suppl):1-10.
68. Gilliam D, Chesters R, Attrill D, et al. Dentine hypersensitivity-guidelines for the management of a common oral health problem. Dent Update. 2013;40
(7):514-524.
To receive 2 credits for this article, log on to www.insidedentistryce.com/go/1425 to take the quiz.
Queries to the authors regarding this CE may be submitted to authorqueries@aegiscomm.com.
About the Author
Howard E. Strassler, DMD
Professor and Director of Operative Dentistry
Department of Endodontics, Prosthodontics, and Operative Dentistry
Baltimore, Maryland