You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Medical advances of all types have made it possible for individuals to live longer and healthier lives. Similarly, as the population ages, more people are taking increasing numbers of medications, polypharmacy, frequently for the treatment of multiple chronic health conditions.1 Polypharmacy is a concern for healthcare providers due to patients' elevated risk of adverse drug reactions, drug interactions, and medication errors.2 Data from the National Health and Nutrition Examination Survey (NHANES) showed an 8% increase in prescription drug use in the United States (U.S.) from 1999-2000 to 2011-2012.3 Additional NHANES data demonstrate that polypharmacy rates increased from 8.2% to 15% over the same period of time.3 Polypharmacy, in combination with off-label drug use, may affect multiple facets of patient care, in medicine and dentistry alike.
While controversy exists on the use of drugs for off-label therapies related to prescribing practices, increased adverse events, and lack of supporting evidence for off-label prescribing, the U.S. Food and Drug Administration (FDA) stated it "recognizes that these off-label uses or treatment regimens may be important therapeutic options and may even constitute a medically recognized standard of care."4 Although the FDA acknowledges the potential benefits of off-label drug therapies, the safety, efficacy and approval of drugs being used off-label are not required or monitored by the FDA. A lack of regulatory evidence supporting the benefits and potential risks of drugs used for off-label purposes may contribute to rising adverse events or potentially ineffective treatments and remains a concern among healthcare professionals.5 Adverse drug-drug interactions are especially concerning since polypharmacy is a common aspect of medication regimens. Addition of drugs not thoroughly tested for their off-label indications can further amplify the potential for adverse reactions.
A highly publicized and well-documented example of the association between off-label drug use and the potential for adverse effects was observed with the drug fenfluramine/phentermine (fen-phen). Fenfluramine/dexfenfluramine and phentermine were individually approved by the FDA as appetite suppressants to be used for a short period of time to aid in weight loss.6 Used individually, these drugs were only slightly effective, but when individuals took the drugs together for the off-label use of appetite suppression, they exhibited rapid weight loss. However, an increased number of individuals were also being diagnosed with valvular heart disease. A meta-analysis conducted by Hopkins and Polukodd examined previous data from endocardiographic studies to assess the prevalence of aortic regurgitation (AR) and mitral regurgitation (MR) related to fenfluramine or dexfenfluramine use.7 Findings revealed a strong association between the duration of the fenfluramine/dexfenfluramine drug regimen and AR (p < 0.00001). Similarly, Wadden et al. reported that 30% of female participants in a retrospective clinical study who took fen-phen for 2 years also met the criteria for valvular heart disease.8 The combination drug fen-phen had never received FDA approval, and it was discontinued in 1997 due to the number of individuals who developed heart valve disease.6 Individuals with a history of fen-fen use were screened for AR and MR and those with subsequent valvular disease were recommended to take antibiotic prophylaxis prior to invasive dental procedures.
The use of dietary supplements, including vitamins, minerals, herbs or other botanicals, has increased among teens and adults of all ages in the U.S.9 However, these supplements do not go through the same drug review process as prescription and over-the-counter medications and are not evaluated for safety and efficacy as they are not intended for the treatment, prevention or cure of diseases.10 Dietary supplements are only regulated by the FDA if they have been proven to be unsafe for use.10 Hence, the use of some dietary supplements may be considered off-label.
Dental hygienists in clinical practice not only treat patients who are utilizing drugs for off-label medical purposes, they may also employ drugs/medical devices for off-label indications in the patient care process. For example, Minimal Intervention, MI Paste™ and MI Paste Plus™ (GC America Inc., Saint Alsip, IL) are FDA approved "to be used for cleaning and polishing procedures as part of a professionally administered prophylaxis treatment."11 Secondary indications identified by the FDA state that MI Paste™ "can be used for the management of tooth sensitivity, post scaling, root planing and bleaching and for the relief of dentinal hypersensitivity."11 In 2012, the FDA issued a warning letter to the manufacturers of MI Varnish™ and pastes, stating that they were in violation of the Federal Food, Drug, and Cosmetic Act due to their promotion of these products for off-label purposes including the treatment of xerostomia due to Sjögrens syndrome and penetration/remineralization of sub- surface lesions in the dentition.12
Fluoride varnishes are used off-label in dental settings for anti-caries treatment and are endorsed by the American Dental Association (ADA).13 The FDA-approved indications for fluoride varnish include the treatment of hypersensitivity, sealing of dentinal tubules for cavity preparations or sensitive root surfaces, and as a cavity liner.14 Although the use of fluoride varnish for caries prevention is preferred for young children due to reduced risk for over-ingestion, rapid adherence compared to the traditional four-minute foam and gel applications, and its higher percentage of fluoride content (5% sodium fluoride varnish compared to 1.1% sodium fluoride), use of fluoride varnish as an anti-caries treatment is not approved by the FDA.15 There have been no studies reported in the literature to date identifying whether the off- label use of fluoride varnish is discussed with patients.
Chlorhexidine gluconate 0.12% (CHX) is an antimicrobial oral rinse and skin cleanser approved by the FDA as a preoperative skin preparation, wound and general skin cleanser, surgical scrub and antiseptic hand rinse, dental rinse for treatment of gingivitis, and as an adjunctive therapy for pocket depth reduction in patients with periodontitis.16 Off-label, CHX has been recommended by the ADA for use in caries prevention although research on its efficacy in that capacity has been inconclusive.17,18 Povidone iodine is approved by the FDA as a broad spectrum external antiseptic for the prevention or treatment of topical infections associated with surgery, burns, minor cuts/scrapes, or the relief of minor vaginal irritation. However, it is used off-label in clinical practice for subgingival irrigation to reduce periodontopathic bacteria within periodontal pockets.19
Alpha-lipoic acid (ALA), a natural supplement not regulated by the FDA, has been used for a myriad of indications including the treatment of nerve pain from diabetes or other diseases, facial pain, weight loss, certain eye conditions, high blood glucose, memory problems, and chronic tiredness. In dentistry, ALA has been studied for the treatment of pain associated with burning mouth syndrome.
Cardiac medications, anticonvulsants, and anti-asthmatics are among the most commonly prescribed drugs for indicated conditions as well as for off-label therapies.22 Dental hygienists treat patients taking these medications on a daily basis, in addition to caring for pediatric, elderly, pregnant women and patients with cancer; all common recipients of off-label drug therapies. The provision of safe and comprehensive patient care requires dental hygienists be familiar with medications and the conditions for which they are being used. Reputable databases in which off-label indications may be found, often charge a subscription fee and it is not known if dental hygienists or dentists would support this cost for their practice, or the extent to which this resource is used.21
While the FDA has an established drug review process ensuring the safety and efficacy of drugs marketed in the U.S., recent advancements in evidence-based medicine and dental practice, including some off-label drug therapies, have led to treatments that may be beneficial to patient care.23 Despite the prominence of off-label drug use, safety and ethical considerations continue to be controversial.24,25 Practitioners must rely on less definitive information for accessing evidence and evaluating general and oral considerations for comprehensive dental hygiene care.24,25 The literature regarding specific off-label drug indications and their use is limited and improved strategies and tools are needed to inform clinicians about common, off-label uses of drugs that may pose risks to patients. More specific information in this area of pharmacology may assist dental hygienists with appropriate treatment modifications and assist with early identification of adverse effects or potential medical emergencies.
The purpose of this study was to examine the knowledge, attitudes, and practices of dental hygienists regarding polypharmacy and drugs used for off-label purposes in dentistry; and to identify any differences in knowledge, attitudes and practices based on level of education, years of practice and type of licensure related to polypharmacy and off-label drug use.
Methods
This cross-sectional designed study utilized a knowledge, attitude, and practice (KAP) online survey instrument. Dental hygienists' knowledge, attitudes, and practices were examined in relation to polypharmacy and off-label drug use and compared to their level of education, years of experience, and type of licensure. This study received Institutional Review Board approval (IRB-FY2016-379) from the Human Subjects Committee of Idaho State University. A convenience sample of 316 dental hygienists practicing in California was utilized for this study; 150 dental hygiene members of the Long Beach Dental Hygienists' Association (LBDHA) and 166 members of the Tri-County Dental Hygienists Association (TCDHA) received an email invitation to participate in the study. Inclusion criteria required current dental hygiene licensure by the state of California; dental hygienists with inactive licenses were excluded.
A previously designed and validated KAP survey assessing dental hygienists' KAP to dietary and herbal supplements was shortened and modified, with permission from the authors, to evaluate KAP related to polypharmacy and off-label drug use.26 The survey instrument was pilot tested with six practicing dental hygienists for reliability by a test/retest method and five content experts assessed validity using a content validity index. The pilot tested, revised survey was administered online through Qualtrics® (Provo, UT). The 45-item survey instrument included questions pertaining to demographics (5), knowledge (8), attitudes (14) and practices (18) related to off-label drug use and polypharmacy. Likert-type, multiple choice and ordinal scale questions related to polypharmacy and off-label drugs included topics such as: discussion with patients, knowledge of therapies used in the dental office, knowledge of FDA-approved indications for drugs used in the dental office, and documentation practices. Participants were asked if suspected use of off-label drugs was investigated during reviews of the medical history and if drugs were used for off-label purposes in the dental office.
The LBDHA and TCDHA databases were used to email a cover letter asking for participation, informed consent, and provided an online link to the survey. Three reminder e-mails were sent after the initial e-mail over a 30-day period.
Data were collected online and imported into IBM SPSS Statistics version 23 (Armonk, NY). Participant characteristics were calculated using descriptive statistics and ANOVA was used to assess the differences in knowledge, attitudes and practices based on participants' level of education, years of practice, and type of licensure. Significance was set at p≤0.05 for ANOVA analyses.
Results
Demographics
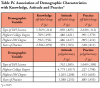
Of the 316 surveys that were emailed, 107 were returned (n=107), yielding a response rate of 34%. The majority of respondents had completed an associate degree for their dental hygiene education (53%) while 42% held a baccalaureate degree as the highest academic degree earned. The majority of participating dental hygienists (72%) practice in a general dentistry setting and 46% practiced for 15 years or less. Professional characteristics of participants are summarized in Table I.
Knowledge
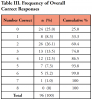
Results of knowledge questions related to off-label drug regulation and drugs used off-label in the dental office are presented in Table II. The mean score for questions answered correctly was 2.28 out of eight. Frequencies for raw knowledge scores (Table III) depict that 25% of participants did not answer any questions correctly, while 74% answered 3 or less questions correctly. ANOVA results of key variables analyzed with relationship to participants' knowledge are depicted in Table IV.
Attitudes
Sixty five percent of participants agreed that informed consent should be obtained when using drugs in the dental office for off-label purposes, and half agreed that off-label prescribing should be illegal. Nearly half (44%) believed that FDA approval for off-label use should be pursued prior to using medications for off-label purposes. A majority of participating dental hygienists (69%) felt confident discussing medications used for off-label purposes with colleagues, while 30% were comfortable answering patient questions, and 41% indicated comfort in initiating discussions. Almost half (48%) of respondents did not feel confident their dental hygiene education prepared them to manage patients using medications off-label and 15% were uncertain. A large majority (85%) felt confident discussing polypharmacy with colleagues and 63% felt confident initiating these discussions with their patients. More than half (66%) of dental hygienists were confident they could inform patients about interactions between commonly used prescriptions and over the counter medications. Sixty-five percent felt confident their dental hygiene education prepared them to manage patients using polypharmacy and 35% were in disagreement or uncertain.
ANOVA results of key variables compiled in Table IV show no significant differences in participant attitudes regarding off-label drugs based on type of licensure, highest degree achieved, or years of experience. However, attitudes regarding polypharmacy differed significantly among respondents based on highest degree earned (p=.011). Dental hygienists with baccalaureate, master or doctoral degrees were more confident initiating discussions with patients and discussing polypharmacy with colleagues. This group also felt better prepared by their dental hygiene education to manage patients utilizing polypharmacy.
Practices
A total of 18 questions pertaining to practices involving off-label medications and polypharmacy comprised this section of the survey. Twenty-six percent of participants reported attending a continuing education course specifically related to medications within the last year. A majority of participants (97%) reported seeing patients who use medications for off-label purposes and 68% identified asking patients about off-label medication use. Thirty percent of the respondents indicated using medications for off-label therapies during patient care and 39% reported explaining this off-label use to their patients. Over two thirds (67%) reported no history of drug interactions with off-label medication use in dentistry and 32% reported no history of any adverse events. All of the respondents reported having patients utilizing polypharmacy. More than half (60%) identified concerns related to adverse events that were related to polypharmacy and almost half (46%) reported concerns related to drug interactions.
Discussion
Results from this study of California dental hygienists indicate an overall lack of knowledge concerning off-label drugs and their use regardless of participants' licensure, level of education, and/or years of experience. Specifically, hours worked and number of patients seen per week had no bearing on knowledge levels; a finding that may be due to content deficiencies in pharmacology, either during dental hygiene education, or later through continuing education courses.
Entry-level dental hygiene programs are required to provide instruction in pharmacology specified in the Accreditation Standards for Dental Hygiene Education Programs mandated by the Commission on Dental Accreditation.27 However, the standards do not specify the amount or type of instruction that should be delivered related to the specific topics in pharmacology, particularly polypharmacy or off-label drug use. Likewise, in the newly revised Compendium of Curriculum Guidelines for Allied Dental Education Programs, pharmacology topics are included but no mention is made of polypharmacy or off-label drug use.28 References to these topics in textbooks is very limited. Depending on the textbook adopted for entry-level dental hygiene programs, inclusion of polypharmacy and off-label drug use is scanty or may not be addressed at all. In the most recent edition of "Basic and Applied Pharmacology for the Dental Hygienist," off-label drug use is defined and discussed early in the text but minimally referenced in chapters related to various pharmacological categories or in dental/dental hygiene settings.29 More in-depth discussions about off-label drug use and polypharmacy and applications to dental hygiene practice should be included as part of a comprehensive pharmacology curriculum for dental hygienists.
Nearly half of respondents reported that their dental hygiene education did not prepare them to discuss off-label drug use with patients. Findings also showed a lack of confidence when answering patients' questions and initiating discussions about off-label drug use, indicating dental hygienists may not be sufficiently prepared upon entering the field of practice. Furthermore, advanced education beyond an associate's degree did not impact the level of knowledge. These findings correspond with a cross-sectional comparison between pharmacy and medical students in the Netherlands regarding knowledge of basic, applied and clinical pharmacology which demonstrated no significant differences in knowledge levels based on number of years of training and education.30 In regards to continuing education following completion of dental hygiene school, only 26% of participants reported that they had attended a course specifically related to medications over the past year. These results indicate all dental hygienists, regardless of their level of education and experience, could benefit from review and expansion of their pharmacology knowledge.
It is possible that in countries where dental hygienists are able to prescribe drugs more emphasis may be given to this area of pharmacology. Dental hygienists in Alberta, Canada may apply for a prescriber identification number after completing a College of Registered Dental Hygienists of Alberta (CRDHA), council approved pharmacy course.31 Course topics include: principles of pharmacology, drugs used in dental hygiene, risk management, medication errors and decision making related to medication use.32 Upon successful course completion, dental hygienists have limited prescriptive authority for antibiotics, antifungal agents, anti-infective agents, antiviral agents, bronchodilators, epinephrine, fluoride, pilocarpine, and topical corticosteroids "for the purpose of treating oral health conditions, providing prophylaxis and treating emergencies."33 While knowledge levels regarding off-label drugs and their uses is unclear, the CRDHA Guidelines Regarding Prescription and Non-Prescription Drugs in Dental Hygiene Practice, clearly states that dental hygienists holding a prescriber ID, "shall not prescribe medications for off-label use unless the drug is part of a research project to investigate use of the drug to treat a documented dental hygiene need. The research project must have received ethics approval from a duly constituted health research ethics board."34 CRDHA guidelines separate prescribing drugs from administering and recommending drugs and while dental hygienists cannot prescribe drugs for off-label use, they may recommend and administer them provided certain requirements are met.
There is no literature appraising off-label drug use and polypharmacy in the discipline of dental hygiene; however, Chen et al. conducted a survey of 350 general practitioners and psychiatrists to address whether or not they were aware of the FDA labeled indications for the drugs they prescribe.35 Results showed that while general practitioners and psychiatrists correctly identified FDA-approved drug indications about 50% of the time, 95% of these same physicians reported knowing the FDA indications of the medications they prescribe and 79% indicated that FDA labeling is an important factor in their prescribing practices. While the knowledge levels among general practitioners and psychiatrists was considerably higher than that of dental hygienists, the findings parallel those of the current study regarding discrepancies in what the medical providers thought they knew and what they were able to correctly identify.
A majority of dental hygienists (70%) indicated that over the past 30 days of practice that they had not used a medication for off-label therapy and 23% noted they used a medication off-label in 1%-13% of patient encounters. Fluoride varnish, considered an off-label anti-caries treatment for use in children, is becoming the common caries prevention treatment for all age groups and is endorsed by the ADA.15,36 However only 15% of participants were able to correctly identify using fluoride varnish for caries prevention as an off-label application, demonstrating a lack of knowledge regarding the indications for fluoride varnish. This finding may have also contributed to the low number of dental hygienists indicating using drugs off-label over the last 30 days of practice.
Participants indicated a familiarity with polypharmacy and indicated the ability to readily identify multiple drug regimens within their patient populations. Unlike off-label medication use, the majority (65%) of respondents felt confident that their dental hygiene education prepared them to manage polypharmacy usage in patient care. It is unclear if this confidence is related to the entry-level curriculum or clinical experiences following completion of dental hygiene education; however, it can be assumed that the ability to more easily detect polypharmacy among patients increases the perceived knowledge of this aspect of pharmacology. Though participants were more confident in discussing polypharmacy, related adverse effects due to polypharmacy were seldom noted. Considering the increased risk of drug-drug interactions and oral side effects associated with polypharmacy, careful assessment of patients' health histories, familiarity with adverse side effects and precautions for each drug are necessary components of total patient care.
Limitations to this study include the representativeness of the sample population. The sample was not randomly chosen, which may have resulted in reduced variation in data. While this survey provided quantitative data offering insight to knowledge, attitudes and practices, it did not produce the kind of data needed to create a full picture of the factors contributing to the low levels that were identified. Additionally, self-reported data cannot be independently verified. Some of the participants did not answer each question, possibly due to lack of knowledge or reluctance to accurately report actual behaviors in the clinical practice setting. A solution for skipping answers, particularly for online surveys, would be to make responses required for advancing to the next question. Subject recall bias should also be considered.
This pilot study points to issues related to knowledge, attitudes and practice concerning polypharmacy and off-label drug use in dental hygiene practice. Further, largescale studies are needed to determine any generalization of the results. In addition, comparative studies among dental hygienists with prescriptive authority and those without may be useful in identifying differences in confidence level, approach to practice, medical history assessment procedures and patient education. Parallel studies regarding dental hygienists' knowledge of off-label drugs used in general medicine may be beneficial in planning for pharmacology courses and continuing education content. Lastly, dental hygiene program curricula and continuing education courses should be examined in terms of the depth and breadth of information provided regarding polypharmacy and off-label drug use.
Conclusion
Health care providers frequently encounter patients practicing polypharmacy and off-label medication use. Results from this cross-sectional study demonstrated dental hygienists in the state of California have limited knowledge related to off-label drug use. Additionally, results indicated no difference in knowledge, attitudes or practices based on type of licensure, highest college degree earned, dental hygiene degree, or years of experience. These findings highlight a need for including increased content in pharmacology in both entry-level dental hygiene programs and continuing education courses for practicing clinicians. More research is needed to identify factors that contribute to a positive increase in knowledge, attitudes and practices in relationship to pharmacological interventions.
About the Authors
Kristen Marie Stephens, RDH, MS is an instructor in the Department of Dental Hygiene, West Coast University, Anaheim, CA.
Tara Johnson, RDH, PhD is an associate professor; JoAnn R. Gurenlian, RDH, MS, PhD is a professor and graduate program director; both in the Department of Dental Hygiene, Idaho State University, Pocatello, ID.
Corresponding author: Kristen M. Stephens, RDH, MS; kstephens@westcoastuniversity.edu
References
1. Köberlein J, Gottschall M, Czarnecki K, et al. General practitioners' views on polypharmacy and its consequences for patient health care. BMC Fam Pract. 2013 Aug 15;14:119-25.
2. Jenny JL, Jenny C, Jayadevan S, et al. Nurses opinion on the attributes of polypharmacy in patient safety. Acta Medica Iranica. 2012 Jul 1;50(7):516-21.
3. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015 Nov 3; 314(17):1818-30.
4. U.S. Food and Drug Administration. Guidance for industry: responding to unsolicited requests for off-label information about prescription drugs and medical devices. [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2011 Dec [cited 2016 Mar 26]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm285145.pdf.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2015 Nov 2;1-9.
6. Gupta SK, Nayak RP. Off-label use of medicine: Perspective of physicians, patients, pharmaceutical companies and regulatory authorities. J Pharmacol Pharmacother. 2014 Nov 20;5(2):88-92.
7. Hopkins PN, Polukoff GI. Risk of valvular heart disease associated with use of fenfluramine. BMC Cardiovasc Disord. 2003 Jun 11;3(5):1-13.
8. Wadden TA, Berkowitz RI, Silvestry F, et al. The fen-phen finale: A study of weight loss and valvular heart disease. Obes Res. 1998 Jul 1;6(4):278-84.
9. Centers for Disease Control and Prevention. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994). [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2011 Apr [cited 2016 Mar 26]. Available from: http://www.cdc.gov/nchs/data/databriefs/db61.htm.
10. National Institute of Health and Human Services. Frequently asked questions [Internet]. Bethesda (MD): National Institute of Health and Human Services: Office of Dietary Supplements; 2013 Jul [cited 2017 Sept 9]. Available from: https://ods.od.nih.gov/Health_Information/ODS_Frequently_Asked_Questions.aspx
11. Lin, CS. (Department of Health and Human Services, Rockville, MD) [Internet]. Letter to: Terry L. Joritz (GC America, Incorporated, Alsip, ILL). 2004 Oct 20. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf4/k042200.pdf
12. U.S. Food and Drug Administration. Inspections, compliance, enforcement, and criminal investigations [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2012 Nov [cited 2016 Apr 8]. Available from https://www.fda.gov/ICECI/EnforcementActions/default.htm
13. American Dental Association Center for Evidence Based Dentistry. Topical fluoride for caries prevention: full report of the updated clinical recommendations and supporting systematic review. [Internet]. Chicago: American Dental Association; 2013 Nov. [cited 2017 Aug 22]. Available from: http://ebd.ada.org/~/media/EBD/Files/Topical_fluoride_for_caries_prevention_2013_update.pdf?la=en
14. U.S. Food and Drug Administration. Premarket notification: Dentsply international. U.S. [Internet]. Silver Spring (MD): Food and Drug Administration; 2012 Oct [cited 2017 Apr 8]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/k122331.pdf
15. Hawkins R, Noble J, Locker D, et al. A comparison of the costs and patient acceptability of professionally applied topical fluoride foam and varnish. J Public Health Dent. 2004 Jun 1;64(2):106-10.
16. Lexicomp. Chlorohexidine gluconate (Lexi-drugs). [Internet]. Lexicomp: Indianapolis, IN: Lexicomp; 2017 Mar [cited 2017 Apr 8]. Available from: http://www.wolterskluwercdi.com/
17. Li Y, Tanner A. Effect of antimicrobial intervention on oral microbiota associated with early childhood caries. Pediatr Dent. 2015 May 1;37(3):226.
18. Rethman MP, Beltrán-Aguilar ED, Billings RJ, et al. Non-fluoride caries preventive agents: full report of a systematic review and evidence-based recommendations. American Dental Association Center for Evidence Based Dentistry [Internet] 2011May. [cited 2017 Aug 22]. Available from http://ebd.ada.org/~/media/EBD/Files/clinical_recommendations_non_fluoride_caries_preventive_agents_full_report.pdf?la=en
19. Perayil J, Menon K S, Fenol A, et al. Comparison of the efficacy of subgingival irrigation with 2% povidoneiodine and tetracycline HCl in subjects with chronic moderate periodontitis: A clinical microbiological study. Dent Res J. 2016 Mar;13(2):98-109.
20. Miziara I, Chagury A, Vargas C, et al. Therapeutic options in idiopathic burning mouth syndrome: literature review. Int Arch Otorhinolaryngol. 2015 Jan;19(1):86-9.
21. Clauson KA, Marsh WA, Polen HH, Seamon MJ. Clinical decision support tools: Analysis of online drug information databases. BMC Med Inform Decis Mak. 2007 Mar 8;7(7):1-7.
22. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006 May 8;166(9):1021-26.
23. Ghinea N, Lipworth W, Kerridge I. Evidence, regulation and ‘rational' prescribing: the case of gabapentin for neuropathic pain. J Eval Clin Pract. 2014 May;21(1):28-33.
24. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012 Oct;87(10):982-90.
25. Kimland E, Odlind V. Off-label drug use in pediatric patients. Pharmacol Ther. 2012 Apr 4;91(5):796-801.
26. Hurlbutt M, Bray K, Mitchell TV, Stephens J. California dental hygienists' knowledge, attitudes and practices regarding herbal and dietary supplements. J Dent Hyg. 2011 Sept 1;85(4):285-96.
27. Commission on Dental Accreditation. Accreditation standards for dental hygiene education programs [Internet]. Chicago: American Dental Association; 2013 [cited 2017 Jan 8]. Available from http://www.ada.org/~/media/CODA/Files/dh.pdf?la=en
28. American Dental Education Association. Compendium of curriculum guidelines (revised edition): Allied dental education programs [Internet]. Washington DC: American Dental Education Association; 2015-2016 May [cited 2017 Jan 8]. Available from http://www.adea.org/BDEBlog.aspx?id=27917&blogid=27619
29. Haveles EB. Applied pharmacology for the dental hygienist. 7th rev. ed. St. Louis: Mosby, Elsevier Inc. 2016. 368 p.
30. Keijsers CJ, Brouwers JR, de Wildt DJ, et al. A comparison of medical and pharmacy students' knowledge and skills of pharmacology and pharmacotherapy. Br J Clin Pharmacol. 2014 Oct 1;78(4):781-88.
31. College of Registered Dental Hygienists of Alberta. HPA frequently asked questions. [Internet]. Edmonton: College of Registered Dental Hygienists of Alberta; 2013 [cited 2016 Dec 23]; [about 2 screens]. Available from http://www.crdha.ca/legislation/hpa-frequently-asked-questions.aspx
32. College of Registered Dental Hygienists of Alberta. Learn more about CRDHA dental hygienist prescribing education [Internet]. Edmonton: College of Registered Dental Hygienists of Alberta; 2015 Dec [cited 2016 Dec 23]. Available from http://www.crdha.ca/media/104984/dh_brochureall_online_feb-2015_final.pdf.
33. College of Registered Dental Hygienists of Alberta. Restricted activities authorization table. [Internet]. Edmonton:2015 Dec [cited 2016 Dec 23]. Available from http://www.crdha.ca/media/223266/restricted-activities-authorization-table-updated-dec-2015.pdf
34. College of Registered Dental Hygienists of Alberta. Guidelines regarding prescription and non-prescription drugs in dental hygiene practice [Internet]. Edmonton: College of Registered Dental Hygienists of Alberta; 2008 Jun [cited 2016 Dec 23]. Available from http://www.crdha.ca/media/1487/crdha-drug-guidelines.pdf
35. Chen DT, Wynia MK, Moloney RM, Alexander GC. U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidemiol Drug Saf. 2009 Jul 8;18(11):1094-1100.
36. American Dental Association. Clinical recommendations for use of professionally-applied or prescription-strength, home-use topical fluoride agents for caries prevention in patients at elevated risk of developing caries. [Internet]. Chicago: American Dental Association; 2013 [cited 2016 Dec 23]. Available from http://ebd.ada.org/en/~/media/ EBD/Files/ADA_Evidence-based_Topical_Fluoride_ Chairside_Guide