You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Microbes within the root canal system are the cause of apical and periradicular periodontitis (endodontic disease). The absence of microbes ensures that apical periodontitis of endodontic origin does not occur. Therefore, the aim of endodontic treatment is to prevent microbial contamination of the root canal system and/or to remove enough microbes to ensure clinical and radiographic success.
A common misconception is that endodontics encompasses only root canal treatment, retreatment, or surgical treatment of post-endodontic disease. Nothing could be further from the truth. A major part of endodontics is maintaining the vital pulp to ensure a healthy periradicular periodontium. Thus, in addition to root canal treatment, indirect and direct pulp capping and pulpotomy procedures are integral parts of endodontic therapy.
Root canal treatment is divided into the microbial control phase (instrumentation, irrigation, intra-canal medication) followed by the filling phase (root and top filling). With both antimicrobial and sealing properties, bioceramic materials are the only materials available in endodontics that contribute to both critical phases for endodontic treatment success.
Bioceramics Defined
Bioceramics are ceramic materials specifically designed for medical and dental use. They include alumina and zirconia, bioactive glass, coatings and composites, hydroxyapetite and resorbable calcium phosphates, and radiotherapy glasses.1-3
Bioceramics are widely used for orthopedic applications (joint or tissue replacement), for coatings to improve the biocompatibility of metal implants, and as resorbable lattices that provide a framework that eventually dissolves as the body rebuilds tissue.4
Bioceramics are classified as:
• bioinert—non-interactive with biologic systems
• bioactive—durable in tissues that can undergo interfacial interactions with surrounding tissue
• biodegradable, soluble, or resorbable—eventually replace or are incorporated into tissues
There are numerous bioceramics currently in use in dentistry and medicine. Alumina and zirconia are bioinert ceramics used in prosthetics. Bioactive glass and glass ceramics are available for use in dentistry under various trade names. In addition, porous ceramics such as calcium phosphate–based materials have been used for filling bone defects. Some calcium silicates (mineral trioxide aggregate [MTA], ProRoot® MTA Root Repair, DENTSPLY Tulsa Dental Specialties, www.tulsadental
specialties.com) and bioaggregates (DiaRoot® BioAggregate, DiaDent, www.diadent.com) have also been used in dentistry as materials for root repair and for apical root filling.
Properties of Endodontic Bioceramics
Endodontic bioceramics are not sensitive to moisture and blood contamination and therefore are not technique sensitive.5 They are dimensionally stable and expand slightly on setting, making them some of the best sealing materials in dentistry.5 When set, they are hard, allowing full compaction of a final restoration, and they are insoluble over time, ensuring a superior long-term seal. When setting, the pH is above 12 due to the hydration reaction, which first forms calcium hydroxide and then dissociates into calcium and hydroxyl ions.6 Therefore, when unset, the material has antibacterial properties. When fully set, it is biocompatible and even bioactive. When bioceramic materials come in contact with tissue fluids, they release calcium hydroxide, which can interact with phosphates in the tissue fluids to form hydroxyapatite. This property may explain some of the tissue-inductive properties of the material.
For the reasons above, these materials are now the material of choice for pulp capping, pulpotomy, perforation repair, root-end filling, and obturation of immature teeth with open apices, as well as for sealing root canal fillings of mature teeth with closed apices.
Available Bioceramic Materials in Endodontics
MTA
Few clinicians realize that original MTA is a classic bioceramic material with the addition of some heavy metals. MTA is one of the most extensively researched materials in the dental field.5,7 It has the properties of all bioceramics—ie, has a high pH when unset, is biocompatible and bioactive when set, and provides an excellent seal over time.
It has some disadvantages, however. The initial setting time is at least 3 hours. It requires mixing (resulting in considerable waste), is not easy to manipulate, and is difficult to remove. Clinically, both gray and white MTA stain dentin, presumably due to the heavy metal content of the material or the inclusion of blood pigment while setting.8,9 Finally, MTA is hard to apply in narrow canals, making the material poorly suited for use as a sealer. Efforts have been made to overcome these shortcomings with new compositions of MTA or with additives. However, these formulations affect MTA’s physical and mechanical characteristics.
Biodentine
Biodentine® (Septodont, www.septodontusa.com) is considered a second-generation bioceramic material. It has properties similar to MTA and thus can be used for all the applications described above for MTA.10 Its advantages over MTA are that it sets in a shorter period of time (approximately 10 to 12 minutes) and it has a compressive strength similar to dentin. A major disadvantage is that it is triturated for 30 seconds in a preset quantity (capsule), making waste inevitable, since in the vast majority of cases, only a small amount is required.
Endodontic Pre-Mixed Bioceramics
Endodontic pre-mixed bioceramic products are available in North America from Brasseler USA (http://brasselerusadental.com) as EndoSequence® BC Sealer™, EndoSequence® BC RRM™ (Root Repair Material™, a syringable paste), and EndoSequence® BC RRM-Fast Set Putty™.
All three forms of bioceramic are similar in chemical composition (calcium silicates, zirconium oxide, tantalum oxide, calcium phosphate monobasic, and fillers), and they have excellent mechanical and biological properties and good handling properties. They are hydrophilic, insoluble, radiopaque, and aluminum free with a high pH, and require moisture to set and harden. The working time of the BC Sealer and BC RRM is more than 30 minutes, and the setting time is 4 hours in normal conditions, depending on the amount of moisture available. The recently introduced EndoSequence BC RRM Fast-Set Putty has all the properties of the original putty but with a faster setting time (approximately 20 minutes). RRM putties and paste are recommended for perforation repair, apical surgery, apical plugging, and vital pulp therapy.
Pre-mixed BC Sealer is the only pure medical-grade bioceramic product available as a sealer for endodontic obturation. It has the same basic chemical composition as the other pre-mixed bioceramic products, but it is less viscous, which makes its consistency ideal for sealing root canals. It is used with a gutta-percha point, which is impregnated on the surface with a nano particle layer of bioceramic. The gutta-percha is used primarily as the delivery device (plugger) (Figure 1 through Figure 3) to allow hydraulic movement of the sealer into the irregularities of the root canal and accessory canals (Figure 4 and Figure 5).
In addition, its surface bonding to the sealer eliminates a critical pathway for coronal leakage of microbes if the coronal restoration has a defective seal. The gutta-percha also is used as a pathway for post preparation or for retreatment if necessary.
To date, more than 50 studies have been performed on pre-mixed endodontic bioceramic materials. The vast majority of these studies have shown that the properties conform to those expected of a bioceramic material and are similar to MTA.
A recent study comparing the results of apicoectomies done with MTA or bioceramic putty on dogs showed the bioceramic putty to be slightly better than the MTA, presumably due to its superior handling properties.11
Indications and Case Examples
Indirect and Direct Pulp Capping and Pulpotomy of Carious Exposures
Historically, endodontists have not recommended vital pulp therapy of cariously exposed permanent teeth. Studies performed in the 1970s showed poor results for this procedure.12,13 However, these studies used calcium hydroxide as the pulp-capping agent and amalgam as the coronal restoration; therefore, if/when the amalgam leaked, the calcium hydroxide base would wash out. This resulted in calcified canals—if the pulp survived—or necrotic pulps with infection and apical periodontitis.
New studies and case series observations have shown that if the base used is antibacterial (such as calcium hydroxide), sets hard, and—most critically—seals well, both indirect and direct pulp-capping and pulpotomy procedures have a very good chance of a successful outcome.14 In relatively young patients, these should be the treatment of choice.
Case 1: Direct Pulp Cap
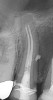
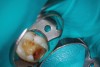
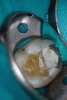
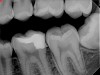
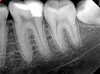
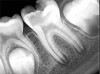
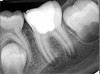
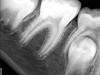
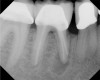
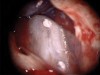
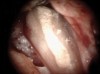
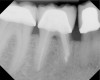
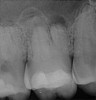
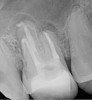
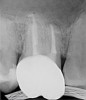
Figure 6 shows the preoperative radiograph of an apparent carious exposure on tooth No. 19 of a 20-year-old male patient. A diagnosis of reversible pulpitis was made based on the history and clinical exam. After anesthesia and caries removal, the exposure was seen (Figure 7) and covered with BC RRM-Fast Set (Figure 8). After the BC base had fully set, a bonded resin was placed and a postoperative radiograph taken (Figure 9). At the 6-month follow-up visit, the tooth was asymptomatic and tested vital. Radiographically, no signs of pathology were noted (Figure 10).
Case 2: Pulpotomy
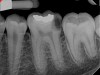
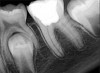
In this case, the tooth tested vital but showed clinical signs of irreversible pulpitis. Treatment with a full pulpotomy was chosen to improve the chances the remaining pulp would survive and remain healthy. The preoperative radiograph in Figure 11 shows extensive caries in the tooth and a slightly widened apical periodontal ligament. A full pulpotomy was performed using the BC putty (Figure 12). After the putty set, a coronal restoration was placed, and an immediate postoperative radiograph was taken and viewed. At the 1-year follow-up, the tooth was asymptomatic, and the radiograph showed continued root development (Figure 13), a healthy apical periodontium, and, importantly, no calcifications in the remaining pulp (as is often seen with a calcium hydroxide therapy). A radiograph taken of the contra-lateral tooth showed similar root development (Figure 14).
Case 3: Apicoectomy and Retrofill
A patient presented with clinical symptoms and radiographic signs of post-endodontic disease (Figure 15). It was determined that the ledge on the mesial canals precluded retreatment and that an apicoectomy was to be performed. After locating the apicoectomy, it was possible to visualize both canals and an obvious isthmus between the two main canals (Figure 16). Retro-preparations were performed in the main canals, and the isthmus between them was grooved and the cavities filled with BC RRM-Putty (Figure 17). At the 20-month follow-up, the patient was asymptomatic and the radiograph showed complete healing (Figure 18).
Root Canal Obturation
Historically, root canal obturation has been the weak link in endodontic therapy. Practitioners have traditionally used a filler (silver points, gutta-percha, resilon) plus a sealer, which provided the barrier to leakage.
Traditional sealers shrink on setting and wash out with time. While some may bond to clean dentin (only), past sealers had no chemical bond to the core material, thus leaving a gap for leakage. To minimize their dimensional instability and limit the effect of wash-out, various methods have been used to minimize the sealer thickness. Cold lateral or warm vertical condensations have been used to maximize the core filler and minimize the sealer thickness. Both have major drawbacks when used with previously available sealers: cold lateral sealers leave too much sealer in the canal irregularities; warm vertical sealers require excessive removal of coronal dentin to fit a plugger to within 4 mm of the apex, thus unnecessarily weakening the root. Also, after the gutta-percha has been heated, it cools and shrinks even more than the sealer. This, plus the fact that the sealer has no chemical bond to the gutta-percha, ensures a gap that provides little resistance to coronal bacterial leakage.
BC Sealer is pre-mixed to a perfect consistency for use as a root canal sealer. It has a high pH that is similar to calcium hydroxide when unset, uses moisture naturally found in the dentinal tubules to set, and is dimensionally stable without washout when fully set. It is not adversely affected by blood or inflammatory exudate, thus minimizing technique sensitivity.
This sealer solves most, if not all, of the previous problems of traditional sealers. Therefore, a thicker layer of sealer can be used because it is dimensionally stable and does not wash out. The core is used only as a hydraulic pump to move the sealer into place and allow for post space preparation or a pathway for retreatment. In addition and most importantly, the root canal preparation can be conservative and better adapted to restorative needs, since a traditional gutta-percha point (and not a metal plugger) is used to move the filling material. An additional benefit of the system is that there are now gutta-percha points that are impregnated with nano particles of bioceramics, so the sealer bonds not only to the root surface but also to the outer surface of the gutta-percha, eliminating the gap between the two (Figure 19 through Figure 21).
Disclosure
Dr. Trope and Dr. Debelian receive royalties from Brasseler USA.
References
1. Best SM, Porter AE, Thian ES, Huang J. Bioceramics: Past, present and for the future. J Eur Ceram Soc. 2008;28;1319-1327.
2. Dubok VA, Bioceramics: Yesterday, today, tomorrow. Powder Metallurgy and Metal Ceramics. 2000;39(7-8);381-394.
3. Hench LL. Bioceramics: From concept to clinic. J Am Ceram Soc. 1991;74(7):1487-510.
4. Hickman K. Bioceramics, Overview. 1999. www.csa.com/discoveryguides/archives/bceramics.php. Accessed September 16, 2014.
5. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190-202.
6. Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35(7):1051-1055.
7. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
8. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
9. Belobrov I, Parashos P. Treatment of tooth discoloration after the use of white mineral trioxide aggregate. J Endod. 2011;37(7):1017-1020.
10. Malkondu Ö, Karapinar Kazandağ M, Kazazoğlu E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int. 2014;2014:160951.
11. Chen I, Karabucak Wang BC, Wang HG, et al. Healing after root-end microsurgery using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. In press.
12. Tronstad L, Mjör IA. Capping of the inflamed pulp. Oral Surg Oral Med Oral Pathol. 1972;34(3):477-485.
13. Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26(9):525-528.
14. Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139(3):305-315.
About the Authors
Martin Trope, DMD
Private Practice
Philadelphia, Pennsylvania
Gilberto Debelian, DMD, PhD
Private Practice, Endodontics
Bekkestua, Norway