You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Over the last 3 decades there has been an explosion of new technology introduced to the field of dentistry. The specialty of endodontics has been particularly impacted by two distinct technological advances that have enabled the trained practitioner to become a better diagnostician and deliver a high level of patient care: the dental operating microscope (DOM) and cone-beam computed tomography (CBCT). The capabilities of these disruptive technologies have enabled practitioners to improve decision-making, especially when adjudicating whether a tooth can be successfully treated or not. These technologies have shifted the way endodontic care is delivered through improved diagnosis and predictable treatment. In addition to DOM and CBCT, the development of advanced cleaning and disinfection systems and techniques is proving to be the latest technologic breakthrough and is initiating another shift in endodontic treatment.
Microscopic Endodontic Techniques
In the early 1990s, Gary Carr, DDS, espoused the advantages of using a DOM (Figure 1) to perform routine endodontic procedures and periapical surgery, although the concept of microscopes in the context of dentistry dates back as early as 1907.1 Carr argued that medical specialties such as otolaryngology, neurosurgery, ear-nose-throat surgery, and microvascular surgery all had similar visual requirements as endodontics and had benefitted from the enhanced visual acuity provided by the operating microscope. As he explained: "Endodontists frequently boast they can do much of their work blindfolded because there is nothing to see. The truth of the matter is that there is a great deal to see, if we only had the right tools" 2 (Figure 2). Carr trained hundreds of endodontists globally, including this author, in microscopic endodontic techniques. Furthermore, he and others developed and patented various instruments to build a new "dental microsurgical world."
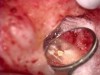
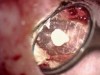
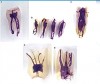
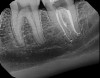
The adoption of the DOM has enabled a well-trained practitioner to treat teeth successfully that in the past might have been considered hopeless.3 These include, but are not limited to, teeth with root and/or furcation perforations, extremely calcified canals, separated instruments in the canals, and failing endodontic treatment. The microscope has also changed the unpredictable nature of apicoectomy procedures and properly addresses intricate root end anatomy (Figure 3 and Figure 4) to ensure success (Figure 5 and Figure 6).2 All in all, the DOM has fundamentally changed the clinical practice of endodontics, and all U.S. endodontic postgraduate programs now require training in microscopic endodontic techniques.4
CBCT Imaging
In 1998, CBCT was introduced in the United States and has since been refined and marketed by several dental/medical imaging companies. The incredible advantage of CBCT 3D imaging over traditional 2D dental imaging has made a significant impact in the clinical practice of orthodontics, implantology, oral surgery, and endodontics.
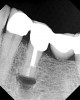
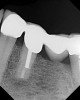
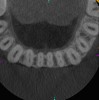
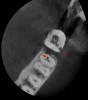
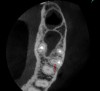
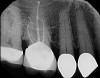
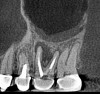
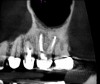
Accurate diagnosis is sometimes the most difficult aspect of daily clinical practice. CBCT has improved the trained practitioner's ability to find hidden periapical pathology, particularly in areas where structures such as the maxillary sinus and zygomatic arch, as well as thick cortical bone, can conceal periradicular lesions (Figure 7 and Figure 8).5-7
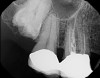
Surveying pulpal anatomy before initiating treatment is another area in which CBCT can benefit a trained operator. Understanding the internal anatomy of the root canal system can help in treatment planning a nonsurgical case, as well as a microsurgical case, to avoid procedural mishaps. CBCT can also facilitate the conservation of tooth and root structure.8 For instance, knowing whether a maxillary molar has a second mesiobuccal (MB) canal can help the practitioner avoid troughing or removing precious dentin when there is no MB2 canal. In addition, more accurately locating the MB2 canal when it is visible on CBCT also assists in conserving tooth structure. The same holds true for a possible lingual canal or additional canal(s) in a mandibular incisor (Figure 9) or bicuspid.
A preoperative CBCT may reduce the need for multiple intraoperative radiographs in complex cases. In previous years, this author acquired a CBCT only on endodontic retreatment cases, surgical cases, and cases where a 2D radiograph suggested complex root anatomy. However, a pretreatment CBCT is now taken on all cases (with the occasional exception of non-retreatment maxillary incisors).
Pretreatment CBCT also assists with diagnosis and treatment when internal and external resorption presents. It is important to know the volume of tooth structure that has been affected by resorption and the exact location of the affected dentin, which can help determine if the tooth is structurally sound enough to initiate treatment, or if extraction is the best option (Figure 10).
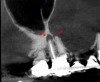
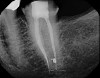
Finally, CBCT can help the clinician diagnose the cause of endodontic failures, especially when untreated canal anatomy is present (Figure 11).9 It can aid in determining whether a nonsurgical or surgical approach is needed. In addition, CBCT imaging is invaluable in surgical treatment planning, particularly if the roots of the tooth approach the maxillary sinus, mandibular canal, or mental foramen. For diagnosing root fractures or cracks, both horizontal and vertical, in this author's experience, root fractures can be seen on most CBCT scans if the crack has separated by at least 75 microns and most vertical root fractures are diagnosed by a narrow pattern of periradicular bone loss along the lateral aspect of a root.
Cleaning Technologies
Cleaning and disinfection of the root canal system is one of the most important (if not the most important) components of successful endodontic treatment. The term "root canal system" (a term originally coined by Dr. Herbert Schilder in 1974) is used because root canal anatomy is not simply a "hollow tube."10 The pulpal anatomy inside a root may be very complex, with multiple apical and lateral foramina, isthmus, "fins," and "web-like" interconnections (Figure 12).11 Traditional approaches have focused on "machining" this anatomy with a rotary file to a size that addresses most, but not all, of this pulpal anatomy, and then administering chemicals such as sodium hypochlorite to dissolve remaining pulp tissue and disinfect the canal dentin, along with ethylenediaminetetraacetic acid (EDTA) to remove the "smear layer."
Contemporary efforts to "clean" the canal are predicated on the use of sonic and ultrasonic activation of treatment chemicals within the canal system, including other devices that actively circulate treatment chemicals within the canal system.12 Unfortunately, "vapor lock," or trapped air within the canal, is a common problem with this technique, preventing treatment fluids from flowing freely.13 Laser activation, or photon induced photoacoustic streaming (PIPS), of treatment fluids within the canal system is another relatively new technology.14 While such efforts have led to improved cleaning and disinfection, some evidence suggests that thorough removal of all tissue, debris, and bacterial biofilm has not been achieved.15 Because these aforementioned techniques can require "machining" of canals to facilitate the flow of treatment fluids throughout the canal system, significant dentin may be removed in the process, which has been associated with poor treatment outcomes.16,17
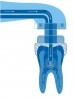
In 2014, FDA clearance was granted to market a "closed loop" canal therapy system (consisting of a console and single-use disposable procedure instrument) that offered broad spectrum acoustic technology and advanced fluid dynamics management. First, an airtight seal is created by the procedure instrument against the tooth (Figure 13). Procedure fluids (diluted sodium hypochlorite and EDTA, along with distilled water) are "degassed" within the console and are constantly refreshed to clean the entire root canal system simultaneously. The stream of fluid entering the tooth creates a powerful shear force, causing hydrodynamic cavitation. The implosion of thousands of microbubbles creates an acoustic field of broadband frequencies that travels through the fluid into the entire root canal system (Figure 14).
With this technology, gentle vortical flow is induced within the root canal system with a slight negative pressure at the apices, which reduces the potential for extrusion. The result is a more complete removal of pulp tissue, bacteria, and bacterial biofilm within the root canal, including debris in anatomically complex structures (eg, isthmi, lateral canals, multiple apical foramina).18-20 In other words, it can clean and disinfect even where a file or rotary instrument cannot reach (Figure 15 through Figure 18). All this is accomplished with minimal instrumentation, most of the time to a size as small as a .04 taper #15 or #20 rotary instrument. In addition, conservation of root dentin results in a stronger root, which may increase the survival rate of the treated tooth.7
Extensive research and testing were conducted prior to the commercial release of the system to establish efficacy and safety. Vandrangi and Basrani demonstrated that this procedure can effectively remove smear layer and penetrate 400 microns into the dentinal tubules of the root canal.19 Charara et al reported no apical extrusion of debris or procedure fluids during the procedure,20 due to a slightly negative pressure at the apical foramen, which prevents extrusion of procedure fluids into the periapical tissues and may improve safety.15 Clinical studies designed to assess healing outcomes are ongoing and initial findings report > 97% of cases are successful after 12 months.21,22
Clinical Observations
After using this procedure for 17 months, this author reports several first-hand observations:
1. Almost all endodontic treatment cases have been converted to a single visit, including (but not limited to) endodontic retreatment cases, complex pulpal anatomy cases such as C-shaped canal anatomy, extremely symptomatic or "hot" teeth, acute abscess cases with hard and soft tissue swelling, and external invasive resorption cases.
2. Patients have little to no postoperative discomfort, no matter the initial condition of the treated tooth, most likely due to the negative pressure system and evacuation of the periapical infection leading to reduced postoperative inflammation.
3. Overall, two thirds fewer rotary instruments are being used in the practice. This results in an economic savings as well as reduced instrument breakage or separation because shaping is smaller (usually no larger than a .04 taper #20). The only reason to shape the canal before the procedure is to facilitate obturation with currently available obturation techniques.
4. Several cases, including endodontic retreatments, have healed in as little as 3-4 months (Figure 19 and Figure 20).
Conclusion
A paradigm shift happens when the traditional methods and attitudes are replaced by new ways and insights. The DOM, CBCT, and multisonic ultracleaning of the canal system each represents an important shift in endodontic practice. As technological advancements continue to drive endodontic science forward, both practitioners and patients will benefit.
About the Author
James A. Smith, Jr., DMD, has practiced the specialty of endodontics for 36 years and currently maintains a private practice in Birmingham, Alabama. He is also an Adjunct Assistant Professor at the University of Alabama at Birmingham School of Dentistry and co-authored an article that appeared in the November 2014 Journal of Endodontics. He lectures nationally to various dental organizations and study groups.
References
1. Bowles SW. A new adaptation of the microscope to dentistry. Dental Cosmos. 1907;49:358-362.
2. Carr GB. Microscopes in endodontics. J Calif Dent Assoc. 1992;20(11):55-61.
3. Kersten DD, Mines P, Sweet M. Use of the microscope in endodontics: results of a questionnaire. J Endod. 2008;34(7):804-807.
4. AAE Special Committee to Develop a Microscope Position Paper. AAE Position Statement. Use of microscopes and other magnification techniques. J Endod. 2012;38(8):1153-1155.
5. Setzer FC, Hinckley N, Kohli MR, Karabucak B. A survey of cone-beam computed tomographic use among endodontic practitioners in the United States. J Endod. 2017;43(5):699-704.
6. Maillet M, Bowles WR, McClanahan SL, et al. Cone-beam computed tomography evaluation of maxillary sinusitis. J Endod. 2011;37(6):753-757.
7. Lofthag-Hansen S, Huumonen S, Gröndahl K, Gröndahl HG. Limited cone-beam CT and intraoral radiography for the diagnosis of periapical pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(1):114-119.
8. Clark D, Kademi J. Modern molar endodontic access and directed dentin conservation. Dent Clin North Am. 2010;54(2):249-273.
9. Hoen MM, Pink FE. Contemporary endodontic retreatments: an analysis based on clinical treatment findings. J Endod. 2002;28(12):834-836.
10. Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18(2):269-296.
11. Tahmasbi M, Jalali P, Nair MK, et al. Prevalence of middle mesial canals and isthmi in the mesial root of mandibular molars: An in vivo cone-beam computed tomographic study. J Endod. 2017;43(7):1080-1083.
12. Van der Sluis LW, Shemesh H, Wu MK, Wesselink PR. An evaluation of the influence of passive ultrasonic irrigation on the seal of root canal fillings. Int Endod J. 2007:40(5):356-361.
13. Tay FR, Gu LS, Schoeffel GJ, et al. The effect of vapor lock on root canal debridement using a side-vented needle for positive-pressure irrigant delivery. J Endod. 2010;36(4):745-750.
14. Divito E, Olivi G. PIPS improving your outcomes using laser activated irrigation. Oral Health. November 2013:62-66.
15. Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of root canal debridement of human molars using the GentleWave™ system. J Endod. 2015;41(10):1701-1705.
16. Wilcox LR, Roskelley C, Sutton T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. J Endod. 1997;23(8):533-534.
17. Rundquist BD, Versluis A. How does canal taper affect root stresses? Int Endod J. 2006;39(3):226-237.
18. Haapasalo M, Wang Z, Shen Y, et al. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod. 2014:40(8):1178-1181.
19. Vandrangi P, Basrani B. Multisonic ultracleaning in molars with the GentleWave™ system. Oral Health. May 2015:72-86.
20. Charara K, Friedman S, Sherman A, et al. Assessment of apical extrusion during root canal irrigation with the novel GentleWave System in a simulated apical environment. J Endod. 2016; 42(1):135-139.
21. Sigurdsson A, Garland R, Le K, Woo S. 12-month healing rates after endodontic therapy using the novel GentleWave™ system: A prospective multicenter clinical study. J Endod. 2016;42(7):1040-1048.
22. Sigurdsson A, Garland R, Le K, Rassoulian S. Healing of periapical lesions after endodontic treatment with the GentleWave procedure: A prospective multicenter clinical study. J Endod. 2018;44(3):510-517.