You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Disclaimer: Participants must always be aware of the hazards of using limited knowledge in integrating new techniques or procedures into their practice. Only sound evidence-based dentistry should be used in patient therapy.
Introduction
This course presents the mechanisms of adverse drug reactions (ADRs), the 30 most common ADRs associated with the top 200 drugs dispensed by U.S. community pharmacies, and less common ADRs that relate to dental therapeutics and/or manifest in the head and neck area. Emphasis is on those ADRs that have a pharmacokinetic or a pharmacodynamic basis and, with the exception of overdose, are the result of drug-drug, drug-food, drug-herbal, and drug-disease interactions with therapeutic doses of drugs.
Please note this is Part I of a two-part series. Adverse Drug Reactions – Part II discusses common immune-mediated and idiosyncratic ADRs related to the top 200 drugs dispensed by U.S. community pharmacies, less commonly noted ADRs affecting oral tissues, and drug-related carcinogenesis and teratogenesis.
Clinicians and patients acknowledge the major role played by drugs in modern healthcare. Understanding how chemicals affect body homeostasis at the molecular level serves as the foundation for developing new therapeutic agents and provides the basis for rational pharmacotherapy. However, drugs seldom exert their beneficial effects without also causing adverse drug reactions (ADRs). This therapeutic dilemma lends credence to the statement that there are no “absolutely” safe biologically active agents.
With the exception of overdose, an ADR is defined by the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA) as “a response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or for the modification of physiologic function.”1,2 The phrase “response to a drug” means that a causal relationship between the therapeutic agent and the ADR is at least a reasonable possibility.
Based on data from the American Medical Association, the incidence rate of ADRs after the administration of FDA-approved drugs is approximately 1 in 123 (0.81%); or 2.2 million per year, 183,333 per month, 42,307 per week, 6,027 per day, 251 per hour, and 4 per minute.3 Death rate extrapolation from the ADRs is 108,000 per year, 9,000 per month, 2,076 per week, 295 per day, and 12 per hour.3 The term “incidence” refers to the annual diagnosis rate, i.e., the number of new cases of ADRs diagnosed each year.
The FDA has one of the most rigorous approval requirements in the world to authorize the marketing of new drugs (Table 1).4,5 However, clinical trials cannot, nor are they expected to uncover every potential ADR. Pre-marketing study cohorts generally include less than 5000 subjects. ADRs that occur at a low frequency can easily be missed. In addition, pre-marketing clinical trials are of relatively short duration. ADRs that develop with chronic use and those that have a long latency period may also escape detection.
Most studies also exclude children, women, and the elderly and are seldom representative of the population exposed to the drug after FDA approval. Consequently, pre-marketing clinical trials detect only those ADRs that occur more frequently than 1 in 1000 subjects, which are then listed in the product's initial labeling (package insert) at the time of approval. In order to have a 95 percent chance of detecting an ADR with an incidence of 1 in 10,000 subjects, a population of 30,000 would have to be exposed to the drug.
ADRs can range from mild to severe illness and can lead to hospitalization, permanent disability, or death. The old term “side effect” as used in the past, described not only negative (unfavorable) reactions; but, at times, concurrent positive (favorable) effects as well. The FDA recommends that this term no longer be used and should not be regarded as synonymous with ADRs.2 Oral healthcare providers should be aware of the spectrum of ADRs and be actively involved in monitoring for and reporting such drug effects.
ADRs may be explained by one of five mechanisms: (1) “on-target” adverse reactions, (2) “off-target” adverse reactions, (3) cytotoxic reactions, (4) immune-mediated reactions, and (5) idiosyncratic reactions, i.e., reactions of unknown mechanisms.6 A drug or drug class may produce toxic or adverse reactions by one or several of these mechanisms. In Adverse Drug Reactions – Part I, the discussion is limited to mechanisms (1), (2), and (3). Mechanisms (4) and (5) are discussed in Adverse Drug Reactions – Part II.
“On-target” Mechanisms of ADRs
Drugs are intended to interact with specific receptors (intended receptors) in specific tissues (intended tissues). An “on-target” ADR may occur when a drug interacts with its intended receptor in the intended tissue.6 With the exception of overdose, therapeutic doses of drugs may result in suboptimal or exaggerated responses because of drug-drug, drug-food, drug-herbal, and drug- disease interaction-related induction or inhibition of pharmacokinetic or pharmacodynamic processes.
Many drug targets are expressed in more than one cell type. An “on-target” ADR may also occur when a drug interacts with its intended receptor, but in an unintended tissue.6 For example, intended targets for H1-histamine receptor antagonists are found in peripheral tissues, i.e., vascular smooth muscle, vascular endothelial cells, lungs, and nerve fibers. However, H1-histamine receptor antagonists with high lipid solubility cross the blood brain- barrier, interact with H1-receptors in the brain, and cause drowsiness.
A relevant example for oral healthcare providers relates to the use of local anesthetic agents (LAs) intended to block sodium channels in neuronal tissues at or near the site of its administration. However, an overdose; or rapid absorption, unintentional intravascular injection, low plasma protein binding, and slow metabolism or clearance of LAs can lead to high plasma levels. This can cause sodium channel blockade in the heart and cardiac depression-induced ventricular arrhythmia, atrioventricular block, and cardiac arrest.
“Off-target” Mechanisms of ADRs
Very few drugs are completely selective; consequently, they can interact with different receptor types. An “off target” ADR can occur when a drug interacts with an unintended receptor either in an intended or in an unintended tissue.6 For example, a β2-adrenergic receptor agonist used to treat asthma may interact with β1-adrenergic receptors in the heart and increase heart rate; and β1-adrenergic receptor blocking agents targeting the heart may also antagonize β2-adrenergic receptors in lungs and cause bronchoconstriction.
Two other examples of “off-target” ADRs, uncovered during post-marketing surveillance, resulted in the withdrawal of both drugs from the market. Terfenadine, an H1-histamine receptor blocking agent also interacted with unintended receptors (potassium channels) in an unintended tissue (heart) that caused fatal cardiac arrhythmias.6 The anorectic agent, fenfluramine, targeting 5-HT serotonin receptors in the brain also interacted with 5-HT2B receptors in the heart causing myofibroblast proliferation and fatal valvular damage.6
Cytotoxic Mechanisms of ADRs
Most drugs undergo metabolism into inactive metabolites in the liver and/or other tissues. Other drugs are pro-drugs, they must be metabolized into active metabolites to produce an effect; subsequently, these may undergo further metabolism into inactive metabolites. In some cases, however, the metabolites are unstable or reactive. For example, therapeutic doses of acetaminophen (APAP) are metabolized by conjugation into APAP-glucuronide and APAP- sulfate; these compounds are nontoxic and are readily excreted.
A small percentage of APAP undergoes oxidation by CYP450 isoenzyme 2E1 into N-acetyl-p-benzoquinoneimine (NAPQI), a highly reactive metabolite. This toxic metabolite must undergo conjugation by glutathione. The APAP-glutathione conjugate is nontoxic and is readily cleared from the body. However, with supratherapeutic dosages glutathione stores are depleted. As NAPQI accumulates, it attacks cellular and mitochondrial proteins in the liver.6 This, in a dose-dependent manner may lead to hepatic fibrosis or necrosis.
The main mechanisms responsible for fibrosis or necrosis include an oxidative pathway, which leads to the formation of reactive oxygen species (ROS) and a reductive pathway, which leads to the formation of reactive nitrogen intermediates (RNI).6 Moderate doses of a toxic drug or metabolite activate mechanisms that result in programmed cell death (apoptosis) and tissue fibrosis. High doses of a toxic drug or metabolite activate mechanisms that lead to uncontrolled cell death and tissue necrosis.
Clinical Frame of Reference Related to ADRs
Two or more drugs administered in therapeutic concentrations at the same time or in close sequence may act independently, may interact to increase or diminish the effect of one or more drugs, or may act to cause an unintended effect. All “on-target,” “off-target,” and cytotoxic ADRs have a pharmacokinetic or a pharmacodynamic basis and include those ADRs which, with the exception of overdose, are precipitated by drug-drug, drug-food, drug- herbal, and drug-disease interactions with therapeutic doses of a drug.6
The risk of ADRs depends on the margin of safety between the dose of a drug required for efficacy and the dose that causes ADRs.6 When the margin of safety is large, ADRs result primarily from overdose; when the margin of safety is small, ADRs may manifest at therapeutic doses. In addition to the dosage of the drug administered and drug-drug, drug-food, drug-herbal, and drug-disease interactions the margin of safety is also modulated by other patient-related variable such as genetic determinants and age.6
Overdose
Drug overdose occurs when a person takes more than the medically recommended dose of a prescription or over-the-counter drug. Overdose may result when a young child or an adult with impaired mental abilities accidentally ingest a medication left within their reach. An adult, especially seniors or people taking multiple medications, can mistakenly ingest the incorrect medication or take the wrong dose of a medication. Some individuals may also be hyperreactive to a medication and even a therapeutic dose may be toxic.
Pharmacokinetic Drug-drug Interactions
Duration and intensity of drug action in both target and non-target tissues is predicated on the drug's plasma level and ability to reach intended or unintended receptors. In addition to dosage, the plasma level of a drug is modulated by the drug's rate of absorption, distribution, metabolism, and clearance.6 These rates may be altered, i.e., induced or inhibited by concomitant drug therapy (Table 2). It is of note that some pharmacokinetic drug-drug interactions may at times be harnessed to optimize a drug's therapeutic effect.
Consider the pharmacokinetic ADR between opioid analgesics and APAP. The opioid by binging to μ-receptors in the gastrointestinal tract increases the tone of the anterior portion of the stomach, decreases gastric motility, and delays the absorption of APAP, which takes place in the intestine. Another example, which affects metabolism, is the interaction between metronidazole and alcohol. Metronidazole inhibits the oxidation of an intermediary toxic metabolite of alcohol and causes severe nausea and vomiting.
Pharmacodynamic Drug-drug Interactions
To produce an effect in target and non-target tissues, a drug must be able to form a complex with its intended and/or unintended receptor. The intended or unintended effect produced by a given plasma level of a drug may result from chronic use or the presence of one or more drugs that lead to (1) changes in the number of available receptors or their ability to respond; or lead to (2) pharmacological, (3) physiological, and (4) chemical drug interactions, which at times may also be used to therapeutic advantage (Table 3).6
Consider the pharmacodynamic ADR between NSAIDs and antihypertensive agents. The inhibition of prostaglandin synthesis by NSAIDs increases vascular tone, which decreases the efficacy of antihypertensive drugs. Another example is a pharmacological drug-drug interaction between epinephrine and β1-adrenergic receptor blocking agents. Since the β1-adrenergic receptors are blocked, unopposed α1-adrenergic receptor activation by epinephrine potentially can result in a hypertensive reaction.
Finally, note two examples of beneficial drug-drug interactions. Epinephrine activates α1-adrenergic-receptors causing vasoconstriction, delaying the systemic absorption of LAs, and increasing LAs' duration of action. Phentolamine mesylate, a competitive α1-adrenergic-receptor antagonist, when injected at the site of LA administration reverses the action of epinephrine as a function of its concentration causing vasodilation, increasing the rate of systemic absorption, and shortening the duration of soft tissues anesthesia.
Drug-food Interactions
Nutrients can act as mechanical barriers and protect the gastric mucosa from irritants; but they can also prevent drug-access to mucosal surfaces and reduce or slow the absorption of some drugs. Other mechanisms such as chemical interactions or chelating reactions with food components can produce inactive complexes. Conversely, a meal with high fat content may actually increase the absorption of some lipid-soluble drugs.
Drug-herbal Interactions
Components of grapefruit inhibit CYP450 isoenzyme 3A4 and increase the plasma level of a number of drugs; for example, it can significantly enhance the anticoagulant effect of warfarin.6 Ginkgo biloba inhibits platelet function; its use with acetylsalicylic acid (ASA), other nonsteroidal antiinflammatory agents (NSAIDs), and clopidogrel, which also interfere with platelet aggregation, increases the risk of bleeding.6
St. John's wort induces the CYP 450 isoenzyme 3A4 and decreases the efficacy of many drugs, including some prescribed by oral healthcare providers, e.g., benzodiazepines and macrolides antibacterial agents.7 Large doses of vitamin C acidify the urine, inhibit the excretion of weak acids such as NSAIDs, acetaminophen, and tetracyclines (e.g., doxycycline and minocycline) and increase the plasma levels of these and other drugs.
Drug-disease Interactions
Cardiac diseases can often result in reduced metabolic activity in general because of poor hepatic and renal perfusion.7 Hepatic dysfunction can affect drug metabolism and biliary excretion.7 Renal insufficiency can be expected to impair renal drug metabolism and clearance.7 Uncontrolled hypothyroidism decreases the rate of drug metabolism and increases sensitivity to CNS depressants; and uncontrolled hyperthyroidism can increase the rate of drug metabolism and increases sensitivity to CNS stimulants.7
Cytotoxic Reactions
Depending on the severity of the toxic insult cytotoxicity may manifest as apoptosis - programmed cell death; or necrosis - uncontrolled cell death.6 Apoptosis is genetically directed self-destruction of DNA-damaged cells and, depending on the regenerating capacity of the tissue, may lead to fibrosis.6 If the toxic insult is severe, programmed cell death cannot be accomplished and the cells undergo necrosis.6 Necrosis is characterized by autolysis of damaged cells, inflammation, and damage to nearby healthy tissues.
Clinical Manifestations of ADRs
The Council of International Organizations of Medical Sciences (CIOMS) in their publication “Reporting Adverse Drug Reactions: Definitions of Terms and Criteria for Their Use” established guidelines for the diagnosis of ADRs and basic requirements for standardized reporting.8 This is especially relevant since most reporting of ARDs is the result of spontaneous monitoring of single cases. The CIOMS codified ADRs under 21 major headings (Table 4) and defined 179 conditions considered reportable.
This discussion is based primarily on the 30 most common ADRs that can occur with therapeutic doses of drugs in the top 200 dispensed by U.S. community pharmacies.9 The top 200 drugs are associated with 9829 individual potential ADRs. The 30 most common ADRs were determined by multiplying each potential ADR by the prescription volume of each drug in the top 200 (Table 5). In addition, less common ADRs that relate to dental therapeutics and/or manifest in the head and neck area are presented.
DailyMed is the official website for FDA-approved label (package insert) information.10 The website provides a standard, comprehensive, up-to-date, look-up-and- download resource for package inserts. The labeling information on the website is the most recent submitted to the FDA by pharmaceutical companies. The information is formatted to make it easy to read, includes strengthened warnings undergoing FDA review, and it is a reliable resource for information on potential ADRs related to specific drugs.
Overdose
Generally, with overdose, the effects of drugs are exaggerated, ADRs become more pronounced, and other, unexpected reactions may be observed. Large overdoses of some medications may cause only minimal ADRs; yet, with other medications even smaller overdoses can cause severe toxicity, including death. A single dose of some medications can be lethal to a young child. Although clinical manifestations of drug overdose vary, signs and symptoms in Table 6 are suggestive of medication-induced toxicity in general.
The U.S. is in the midst of an opioid overdose epidemic. Opioid analgesics and heroin killed more than 33,000 people in 2015, more than during any other year on record. Nearly half of all opioid overdose deaths were related to prescription opioid analgesics.11 An opioid overdose can reliably be identified by the presence of three clinical signs and symptoms referred to as the “opioid overdose triad”: (1) pinpoint pupils (miosis), (2) unconsciousness, and (3) respiratory depression (less than 12 breaths/min).12
Combining opioid analgesics with alcohol and other central nervous system (CNS) depressants increases the risk of respiratory depression and death. Indeed, opioids, alcohol, and sedatives are often present in fatal drug overdose. Risk factors for overdose with prescribed opioid analgesics include a history of substance abuse, high prescribed dosage, male gender, older age, mental health conditions, concurrent prescriptions of other CNS depressants (e.g., benzodiazepines), and lower socioeconomic status.
ADRs Affecting Oral Tissues
The CIOMS does not provide a specific code for ADRs associated with oral tissues; however, it does include stomatitis and ulcerative stomatitis under the category of ADRs related to the gastrointestinal system.8 In addition, xerostomia (ranked #28) and taste disturbances (ranked #30) are among the 30 most common ADRs identified with the top 200 drugs dispensed by U.S. community pharmacies.9 Included under this heading are those orally-related ADRs that are of special interest to oral healthcare providers.
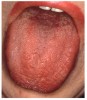
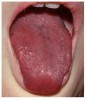
Many drugs can cause xerostomia (Table 7).13-16 Reduced salivary flow may be related to a drug's parasympatholytic or antimuscarinic effect in the CNS at parasympathetic and some sympathetic ganglia, or at parasympathetic and some sympathetic effector junctions. Other drugs cause fluid and electrolyte imbalance; glandular vasoconstriction; or alter fluid movement from plasma through acinar cells to the ductal system and, ultimately, into the oral cavity (Figure 1 and Figure 2).
Many drugs, e.g., chlorhexidine, metronidazole, benzodiazepines, oral hypoglycemic agents, angiotensin converting enzyme (ACE) inhibitors, diuretics, amiodarone, calcium-channel blocking agents, and H1-histamine receptor antagonist have been implicated in dygeusia or taste alterations characterized as bitter or metallic.13-15 The mechanisms of action of these drugs related to taste alterations are poorly understood, but appear to be associated with drug effects on trace metals (e.g., zinc) in plasma membranes.
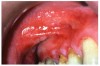
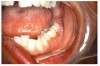
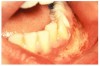
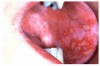
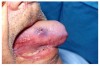
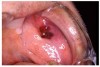
Stomatitis and ulcerative stomatitis represent cytotoxic reactions to topically applied agents, e.g., LAs; or may result from the systemic administration of cytotoxic drugs, e.g., antineoplastic agents, which damage not only tumor cells, but all rapidly dividing normal cell populations.14,16 The degree of tissue damage depends on the specific agent, dosage, dosage schedule, and patient-related variables. The lesions may appear as erythematous macules, patches, papules, plaques, or diffuse ulcerations (Figure 3 and Figure 4).
Antibacterial and corticosteroid therapy is often complicated by superinfection with candidal organisms in oral tissues.14,16 Antibacterial agents kill bacteria allowing candidal species to successfully compete for nutrients. Corticosteroids promote gluconeogenesis and a hyperglycemic state facilitates the growth of the opportunistic candida species. Candidiasis may appear as white, raised, cottage cheese-like, or pseudomembranous lesions that can be scraped off, leaving a red and sometimes hemorrhagic base.
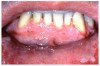
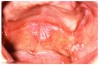
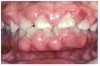
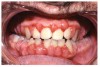
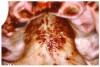
Gingival overgrowth may be associated with the administration of calcium-channel blocking agents, phenytoin, and cyclosporine.13-16 The mechanisms responsible are unclear, but they appear to be related to altered calcium metabolism and concomitant poor oral hygiene-related inflammation.13While the enlarged tissue is usually firm and painless, it may interfere with mastication; and, with significant inflammation, the patient may report pain and gingival bleeding (Figure 9 and Figure 10).
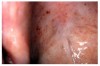
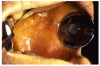
ASA and other NSAIDs acetylate cyclooxygenase and inhibit platelet thromboxane A biosynthesis; clopidogrel inhibits adenosine diphosphate receptor-mediated platelet activity; and other medications such as antineoplastic agents may induce profound thrombocytopenia.13,15 Clinical manifestations of platelet-related bleeding diatheses include petechiae (Figure 11), purpura (Figure 12), ecchymosis (Figure 13), spontaneous gingival bleeding, and increased potential for perioperative bleeding.
Oral anticoagulants (e.g., warfarin which inhibits vitamin K-dependent clotting factors, primarily Factor VII) and the parenteral anticoagulant heparin (which inhibits Factors II and X) reduce clot formation.13 Clinical manifestations of anticoagulant-related bleeding diatheses include hemorrhage, which may be spontaneous or precipitated by trauma. Oral manifestations may include spontaneous gingival bleeding (Figure 14), submucosal bleeding with hematoma formation, and increased peri- and post-operative bleeding.
Prevention and treatment of osteoporosis include the administration of antiresorptive agents such as bisphosphonates (BPs). A rare ADR related to BPs is medication-related osteonecrosis of the jaw precipitated by dentoalveolar trauma.17 When BP molecules are released from the bone matrix some are internalized by osteoclasts resulting in inhibition of the mevalonate pathway essential for the synthesis of signaling proteins to activate osteoblast precursors. BPs also inhibit angiogenesis and are toxic to soft tissues.
ADRs Affecting the Gastrointestinal System
Nausea, vomiting, diarrhea, constipation, abdominal pain, dyspepsia, and anorexia are among the 30 most common potential ADRs associated with therapeutic dosages of the top 200 drugs dispensed by U.S. community pharmacies.9 CIOMS does not list nausea and vomiting and considers abdominal pain an undesirable term to describe ADRs. When a patient complains of abdominal pain CIOMS recommends considering drug-induced colitis, pancreatitis, and peptic ulcer disease in the differential diagnosis.8
The physiologic purpose of nausea is to prevent food intake; that of vomiting is to expel food or other toxic substances present in the upper part of the gastrointestinal tract.18 Nausea and vomiting occur in response to activation of the vomiting center either directly or through the chemoreceptor trigger zone (CRTZ). The CRTZ is outside the blood-brain barrier and is accessible to drugs circulating in the vascular compartment. NSAIDs, antibiotics, opioids, digitalis, and antineoplastic agents are examples of emetic drugs.18
Diarrhea and associated fecal urgency and incontinence may be defined as passage of loose, unformed stool with increased frequency.9,19 Chronic diarrhea may be due to lactose intolerance, inflammatory bowel disease, malabsorption syndromes, endocrine disorders, irritable bowel syndrome, and the abuse of laxatives.19 Common causes of acute diarrhea include viral and bacterial infections; and drugs such as antibacterial agents, Mg-containing antacids, antineoplastic agents, and colchicine.19
Constipation may be defined as the passage of excessively dry stool, infrequent stool, or stool of insufficient size.9,19 It involves the subjective sensations of incomplete emptying of the rectum, bloating, flatus, lower abdominal discomfort, anorexia, malaise, headache, weakness, and giddiness. Common causes of acute constipation include antihistamines, opioid analgesics, neuroleptics, antidepressants, anticonvulsants, aluminum- and Ca-based antacids, Ca-channel blocking agents, and anti- parkinsonian drugs.19
Abdominal pain is any pain in the topographical area of the abdomen. When describing an ADR, its relation to anatomical site and other symptoms should be specified.20 For example, drug-induced pancreatitis is usually an acute condition characterized by upper abdominal pain accompanied by severe nausea and vomiting.9,21 Some medications implicated in causing acute pancreatitis include corticosteroids, diuretics, oral hypoglycemic agents, estrogens, anticonvulsants, and antineoplastic agents.21
The principal symptom of peptic ulcer disease is also abdominal pain. Vomiting may also occur and the patient is predisposed to hemorrhage and perforation.9,22 Hemorrhage may vary from slight bleeding to massive hemorrhage and the patient may vomit variable quantities of blood. If the blood goes down into the intestines, a large amount of altered blood makes the stools black and tarry (melena). The ulcerogenic effect of NSAIDs is well established and is attributable to inhibition of prostaglandin synthesis.22
Of particular concern to oral healthcare providers should be the patient complaining of lower abdominal pain, acute diarrhea with blood in the stool that is currently taking or has been prescribed in the recent past clindamycin or a broader spectrum penicillin or cephalosporin. The signs and symptoms are likely due to bacterial superinfection, e.g., an overgrowth of C. difficile in the gastrointestinal tract, a serious presentation of which is pseudomembranous colitis.9
Dyspepsia is a sensation of discomfort or pain in the upper abdomen below the sternum. Patients may describe it as heartburn or indigestion and complain of feeling nauseous and bloated after eating, regurgitating (burping) food or liquid.18 Drugs associated with acute dyspepsia include NSAIDs, opioids, antibacterial agents, oral hypoglycemic agents, ACE-inhibitors, angiotensin II-receptor antagonist, corticosteroids, estrogens, anti-parkinsonian drugs, digoxin, niacin, fenofibrate, and SSRIs.18
Anorexia is defined as lack or loss of appetite for food.8,9 It is to be differentiated from lack of appetite related to nausea, vomiting, or diarrhea; or hyporexia, decreased appetite; or anorexia nervosa, which is characterized by refusal to maintain normal body weight. With anorexia, limitation of food-intake and weight loss is not deliberate. Drugs that may have inhibitory effects on appetite and food intake include certain antibacterial agents, antineoplastic agents, fluoxetine, digoxin, quinidine, hydralazine, and vitamin A.
ADRs Affecting the Liver and Biliary System
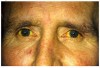
A potential complication of nearly every medication is hepatocellular liver injury.9 One such drug discussed at length earlier is APAP.6 During the past decade it has been identified as the leading cause of acute liver failure (ALF) in the United States and up to 50% of APAP-related cases of ALF are due to unintentional overdose.23 Clinical signs and symptoms of APAP overdose include nausea, vomiting, abdominal pain, anorexia, and a few days later, elevated bilirubin presents as jaundice (Figure 15 and Figure 16).24
In response to the problem of APAP-related overdosing, the FDA called upon healthcare professionals to discontinue prescribing combination drug products with more than 325 mg of APAP per formulation.23 Ultimately, the FDA and the pharmaceutical industry have taken action to protect consumers from the risk of ALF by formally withdrawing from the market all prescription combination analgesics containing more than 325 mg of APAP per unit dose.23 APAP, 650 mg per dose, q4h, may still be prescribed.
ADRs Affecting the Ears
Dizziness is the most common potential ADR associated with therapeutic dosages of the top 200 drugs dispensed by U.S. community pharmacies.9 It is an imprecise term describing various related sensations such as faintness, a feeling of impending syncope; light-headedness; and a feeling of imbalance.25 A false sensation of movement of self or the environment is called vertigo.8,25 Because these symptoms overlap patients often use the terms dizziness and vertigo interchangeably.
Ototoxic drugs that affect the vestibular apparatus result in vertigo; cochlear ototoxicity results in hearing loss.8 Ototoxic drugs may also cause tinnitus described by patients as noise in the ears such as buzzing, ringing, roaring, whistling, hissing, or pulsating.8,25 Potentially ototoxic drugs include salicylates; antibiotics such as vancomycin, metronidazole, clindamycin, aminoglycosides (e.g., gentamycin and tobramycin), and macrolides; diuretics (e.g., furosemide); and antineoplastic agents (e.g., cisplatin).25
ADRs Affecting the Cardiovascular System
Hypotension is the most common ADR related to the cardiovascular system.9 It is defined as blood pressure (BP) below normal for an individual. Postural hypotension is a decline from baseline of ≥ 20 mm Hg in the systolic BP and/or a decline of ≥ 10 mm Hg in the diastolic BP with symptoms of cerebral ischemia (syncope) following postural change.8,26 Common drugs that produce postural hypotension include diuretics, α1 and β1-receptor blockers, nitrates, digitalis, ACE-inhibitors, and calcium channel blockers.26
Syncope is defined as sudden brief loss of consciousness due to cerebral ischemia.8,26 In a young adult it is usually precipitated by a generalized, progressive autonomic discharge secondary to anxiety, pain, heat, or humidity. The initial adrenergic response to a stressor is followed by an overwhelming cholinergic surge just prior to unconsciousness. Syncope in patients over 50 years of age may likely be secondary to cardiovascular disorders (e.g., dysrhythmia, postural hypotension), hypoglycemia, or cerebrovascular insufficiency.26
Palpitation is a general term used by patients to describe an unpleasant awareness of forceful, rapid, or irregular heartbeat.8,26 Arrhythmia is defined as either a pulse rate < 60 beats/min or a pulse rate > 100 beats/ min, the rhythm may be regular or irregular.8,27 Arrhythmias are frequently associated with palpitation, syncope, dyspnea, angina pectoris, heart failure, or cardiogenic shock. Arrhythmia may result from drug-induced abnormal impulse generation or abnormal impulse conduction in the heart.27
Digoxin, a drug used to treat congestive heart failure, atrial flutter, and atrial fibrillation is also associated with causing cardiac arrhythmias. Macrolide antibiotics are known to cause cardiac arrhythmias characterized by prolongation of the QT interval, palpitations, hypotension, and chest pain. Macrolides-induced QT interval prolongation is amplified in combination with azole antifungal agents, some calcium channel blockers, and protease inhibitors because of CYP450 isoenzyme 3A4 inhibition.27
ADRs Affecting the Respiratory System
Dyspnea is ranked #17 among the 30 most common ADRs associated with the top 200 drugs dispensed by U.S. community pharmacies.9 It is described as shortness of breath, breathlessness, inability to take a deep breath, suffocating, cannot get enough air, or pain on breathing.6,8,28 Dyspnea is to be distinguished from impairment of expiratory airflow associated with chronic obstructive pulmonary disease.28 Dyspnea may be caused by opioids, β1-adrenergic receptor agonist, and intolerance to ASA, NSAIDs, and adenosine.
ADRs Affecting the Urinary System
The kidneys provide the final common pathway for the clearance of most drugs and their metabolites. Consequently, they are exposed to high concentrations of potentially toxic metabolites that can cause renal damage, especially in the presence of pre-existing renal disease.6 For example, the chronic use of NSAIDs can lead to direct nephrotoxicity called analgesic nephropathy, related to the inhibition of prostaglandin synthetase resulting in reduced glomerular blood flow and acute renal failure.6,8,29
Urinary incontinence is the involuntary loss of urine. Diuretics, alcohol, and caffeine increase urine production leading to increased frequency, urgency and nocturia.30 Ca-channel blocking agents, anticholinergic drugs, alpha-adrenergic agonists cause urinary retention and overflow incontinence.30 Opioid analgesics can cause urinary retention, and like psychotropic drugs such as benzodiazepines, antidepressants, and antipsychotic agents may affect incontinence by blunting awareness to void.30
ADRs Affecting the Neuropsychiatric System
Somnolence, sleepiness or drowsiness, is a symptom characterized by a state of strong desire for sleeping or sleeping for unusually long periods.31 The use of alcohol and street drugs, such as marijuana and heroin, can cause drowsiness.31 Somnolence may also be caused over-the-counter medications such as antihistamines; and prescription drugs such as anxiolytic agents, muscle relaxers, opioids analgesics, and sleeping pills.31 Drowsiness can also be caused by some herbal teas and supplements, such as valerian.31
Insomnia is a symptom characterized by difficulty in falling to sleep or to stay asleep. It is most often caused by psychiatric disorders, i. e., mood disorders and anxiety.31 Drug-related insomnia may be associated with chronic use of drugs or result from drug withdrawal.31 The use of alcohol, anticonvulsants, antidepressants, and thyroid hormones therapy may interfere with sleep. The withdrawal of CNS depressants such as opioids analgesics, benzodiazepines, alcohol, cocaine, and heroin may also lead to insomnia.
Depression is a mental state dominated by a lowering of mood and it often includes other symptoms such as anxiety, agitation, sleep disturbances, alteration in appetite, and feelings of unworthiness, and suicidal thoughts.8,32 It is a frequent consequence of treatment with β1-adrenergic receptor antagonists, digoxin, benzodiazepines, corticosteroids, levodopa, phenothiazines, and steroids.32 It should be noted, however, that depression may be an appropriate response to transient life-stress situations.
Anxiety is the distressing experience of dread and foreboding; an unpleasant emotional experience characterized by nervousness, uneasiness, and fear.33 Anxiety may be related to medical illnesses, psychiatric illness, or psychological illness. Dietary causes of anxiety include caffeine and monosodium glutamate (Chinese-restaurant syndrome).33 Symptoms of anxiety may be caused by antipsychotic drugs, anticholinergic agents, digitalis, amphetamines, cocaine; as well as withdrawal of alcohol or sedative-hypnotics.33
ADRs Affecting the Peripheral and Central Nervous Systems
Headaches may be primary or secondary.34 Primary headaches include migraine, cluster, and tension headaches. Secondary headaches may be related to extracranial causes (e.g., dental problems, sinusitis, and carotid artery disorders), intracranial causes (e.g., brain tumors and vascular disorders), and exposure to toxins and drugs. Secondary headache may be a symptom of exposure to monosodium glutamate (MSG); analgesic overdose; caffeine withdrawal; and treatment with estrogen and nitrates.34
Fatigue or weakness is a subjective feeling of tiredness and may have physical or mental causes.35 Mental fatigue is due to prolonged periods of cognitive activity. Physical fatigue may be due to normal muscle exertion; or it may be caused by endocrine/metabolic problems, cardiopulmonary abnormalities, psychiatric disorders, vitamin deficiencies, or drug withdrawal.35 Fatigue is common with medications such as antihistamines and β1-adrenergic receptor antagonists.
Tremor is unintentional, rhythmic muscle activity involving to-and-fro movements (oscillations) affecting most commonly the hands, arms, head, face, and legs.36 Tremor may be a symptom of a neurological disorder; it is most often associated with Parkinson's disease. It is also a well-recognized adverse reaction to such drugs as amphetamines, cocaine, thyroid hormones, mercury poisoning, corticosteroids, SSRIs, and alcohol abuse; and alcohol and benzodiazepines withdrawal.36
Tardive dyskinesia (TD) is an ADR related to long-term dopamine2-receptor blockade by antipsychotic drugs such as haloperidol, chlorpromazine, thioridazine, trifluoperazine, fluphenazine, and perphenazine.36 Symptoms include oral dyskinesia, i.e., involuntary muscle movements characterized by continual chewing, intermittent darting of the tongue (fly fishing) and lip-smacking, pursing, or puckering.8 TD is sometimes associated with abnormal, uncontrollable, writhing movements of the arms and/or legs.
Paresthesia is an unpleasant, abnormal sensation of tingling, numbness, or burning usually felt in the hands, feet, arms, or legs, but can also manifest in other part of the body.37 Chronic paresthesia is many times a symptom of an underlying neurological disease or traumatic nerve damage. Drugs associated with paresthesia include LAs, long-term exposure to nitrous oxide, mercury poisoning, heavy metal poisoning, led poisoning, anticonvulsant drugs; and withdrawal of benzodiazepines and SSRIs.37
Fever is elevated body temperature, i.e., > 37.8° C orally.38 It is regulated by the hypothalamic thermoregulatory center that maintains the internal temperature within a maximum fluctuation of 0.6° C. Fever results when something raises the hypothalamic set point and triggers peripheral vasoconstriction to preserve heat and shivering, which increases heat production.38 Drugs that can increase heat production include amphetamines, cocaine, general anesthetics, and antipsychotic agents.38
ADRs Affecting the Endocrine/Metabolic System
The treatment of diabetes mellitus includes daily administration of insulin and/or an oral hypoglycemic agent, which stimulate cellular glucose uptake. Increased rates of insulin absorption associated with increased skin temperature, heavy exercise, anxiety, infection, and pain lead to hypoglycemia.39 Insulin and/or oral hypoglycemic agents in the absence of adequate intake of carbohydrates also lead to hypoglycemia.39 Signs and symptoms of hypoglycemia are epinephrine-, glucagon-, and neuroglycopenia-related.39
Adrenergic manifestations include twitching, tremor, anxiety, nervousness, sweating, pallor, cold and clammy skin, and mydriasis. Glucagon-related manifestations include hunger, boborygmus (rumbling, growling, or gurgling noise emanating from the patient's gut), nausea, vomiting, and abdominal pain. Neuroglycopenic manifestations include fatigue, weakness, lethargy, paresthesia, headache, subtle reduction in mental capacity, impairment of action and judgment, focal or generalized seizure, and coma.
Summary
“On-target,” “off-target,” and cytotoxic ADRs, with the exception of overdose, are related to the use of therapeutic dosages of drugs and are predicated on the same pharmacokinetic and pharmacodynamic pathways as the therapeutic effects of drugs. Prerequisites to considering ADRs in the differential diagnosis of a patient's signs and symptoms include awareness that an ever increasing number of patients are taking more and more medications (polypharmacy) and familiarity with relevant literature about ADRs.
References
1. Leape L, Abookire S, World Health Organization, et al. WHO draft guidelines for adverse event reporting and learning systems: from information to action. Geneva, Switzerland: World Health Organization. 2005.
2. U.S. Food and Drug Administration. Guideline for Industry. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. Accessed June 12, 2017.
3. Mayo Clinic. Statistics about adverse reactions. Prevalence and incidence statistics for adverse reactions. Accessed June 12, 2017.
4. Valhe JL, Hutto DL, Postema M. Drug discovery and preclinical development- Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 4th edition. David E Golan (Ed). Philadelphia, PA: Wolters Kluwer. 2017. 919-932.
5. Goldberg MA, Kuta AE. Clinical drug evaluation and regulatory approval- Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 4th edition. David E Golan (Ed). Philadelphia, PA: Wolters Kluwer. 2017. 933-945.
6. Conner MW, Dorian-Conner C, Vaidya VS, et al. Drug toxicity- Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 4th edition. David E Golan (Ed). Philadelphia, PA: Wolters Kluwer. 2017. 70-86.
7. Guengerich FP. Drug metabolism- Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 4th edition. David E Golan (Ed). Philadelphia, PA: Wolters Kluwer. 2017. 43-55.
8. Council for International Organizations for Medical Sciences. Reporting adverse drug reactions. Definitions of terms and criteria for their use. Accessed June 12, 2017.
9. Roswarski M, Villa KR, Kiersma ME, et al. Prevalence of Adverse Drug Effects/Adverse Drug Reactions in 200 Most Commonly Prescribed Drugs Corrected for Prescription Volume. 2009. Pharmacy Practice Faculty Presentations. 69. Accessed June 12, 2017.
10. National Institute of Health. U.S. National Library of Medicine. DailyMed. Accessed June 12, 2017.
11. Centers for Disease Control and Prevention. Opioid overdose. Accessed June 12, 2017.
12. World Health Organization. Information sheet on opioid overdose. November, 2014. Accessed June 12, 2017.
13. Ciancio SG. Medications' impact on oral health. J Am Dent Assoc. 2004 Oct;135(10):1440-8; quiz 1468-9.
14. Jacobsen PL, Chávez EM. Clinical management of the dental patient taking multiple drugs. J Contemp Dent Pract. 2005 Nov 15;6(4):144-51.
15. Torpet LA, Kragelund C, Reibel J, et al. Oral adverse drug reactions to cardiovascular drugs. Crit Rev Oral Biol Med. 2004 Jan 1;15(1):28-46.
16. Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004 Jul 1;15(4):221-39.
17. American Association of Oral and Maxillofacial Surgeons. Medication-Related Osteonecrosis of the Jaw - 2014 Update. Accessed June 12, 2017.
18. Greenberger NJ. Approach to the patient with upper GI complaints – The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 70-83.
19. Bharucha AE. Approach to the patient with lower GI complains - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 83-93.
20. Ansari P. Acute abdominal and surgical gastroenterology - - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 105-119.
21. Freedman SD. Pancreatitis - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 140-145.
22. Cohen S. Gastritis and peptic ulcer disease - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 128-137.
23. FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. 01/13/2011. Accessed June 16, 2017.
24. Herrine SK. Navarro VJ. Drugs and the liver - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 232-235.
25. Tucci DL. Approach to the patient with ear problems - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 413-428.
26. Tanser PH. Approach to the cardiac patient - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 2017-2047.
27. Mitchell LB. Arrhythmias and conduction disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 2142-2178.
28. Lechtzin N. Approach to the patient with pulmonary symptoms - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1826-1847.
29. McMillan JI. Glomerular disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 2387-2411.
30. Lui PD. Voiding disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 2352-2364.
31. Doghramji K. Sleeping and wakefulness disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1703-1714.
32. Coryell W. Mood disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1538-1552.
33. Greist JH, Jefferson JW. Anxiety disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1493-1501.
34. Silberstein SD. Headache - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1715-1725.
35. Jacewitcz M. Approach to the neurologic patient - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1586-1605.
36. Eidelberg D, Pourfar M. Movement and cerebellar disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1759-1778.
37. Rubin M. Peripheral nervous system and motor unit disorders - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1784-1803.
38. Tunkle AR. Biology of infectious diseases - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1148-1165.
39. Crandall JP. Diabetes mellitus and disorders of carbohydrate metabolism - The Merck manual of diagnosis and therapy, 19th edition. Robert S. Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 866-888.
About the Authors
Kristin A. Williams, DDS, MPH
Dr. Kristin A. Williams is an Assistant Professor, Department of Community Dentistry, and Assistant Dean for Admissions and Student Affairs at Case Western Reserve University, School of Dental Medicine in Cleveland, OH. Dr. Williams received her D.D.S. in 1989, completed a residency in Dental Public Health, and obtained a Master of Public Health in 2005, all at the School of Dental Medicine, Case Western Reserve University. Dr. Williams holds several positions in local professional societies, serves as a reviewer for national public health publications, published extensively in peer-reviewed journals, and presented many scientific programs at local, state and national professional meetings.
Email: kaw14@case.edu
M. Louay Taifour, BDS, DMD
Dr. Taifour earned his dental degree from Beirut Arab University School of Dental Medicine, then completed an AEGD residency and a Restorative Fellowship at Case Western Reserve School of Dental Medicine. Dr. Taifour teaches several pre-doctoral courses and is a full time clinical attending for the AEGD residency program.