You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
In the dental clinic setting, the potential for pathogen transmission from staff to patients (as well as from patients to staff) is of chief concern. Sources of infectious microorganisms include contaminated bodily fluids (eg, saliva and blood), improperly sterilized equipment/instruments, and airborne transmission. The invasive nature of the procedures performed on behalf of patients by both dentists and dental hygienists, coupled with the high degree of potential exposure within the oral cavity, underscores the need for pathogen elimination from all potential sources. Dental hygienists in particular may serve as a vital link in the potential transmission of microbes in a dentistry, as they are in contact with all incoming patients, including those seeking only routine services (eg, cleanings and x-rays) and those who require more complex procedures.
In addition to the aforementioned sources of pathogens in the dental setting, inanimate objects (fomites) may also function as reservoirs for such microorganisms within the indoor environment. In the clinical setting, fomite contamination occurs by touch via infected patients or staff, and from the settling of aerosols (eg, from sneezing) expelled from infected persons. The subsequent touching of contaminated fomites by individuals may then result in the transfer of infectious microorganisms to the hands.1,2 Self-inoculation may then occur when contaminated fingers make contact with the mouth, nose, or eyes,3 with direct transmission and colonization of skin also a possibility for some pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).4 Studies assessing bacterial occurrence in the dental setting have focused primarily on clinical patient-care areas.4-6 However, data with regards to bacterial levels on surfaces commonly found in dental offices not associated with corresponding schools or clinics is largely nonexistent.
The implementation of disinfection-based hygienic interventions in the hospital setting has resulted in lower levels of nosocomial pathogens such as MRSA on surfaces, with corresponding decreases subsequently observed in incident cases among patients.7 MRSA is also a bacterial pathogen of great concern in dental clinics, and has been investigated extensively with regards to aerosol transmission,8 surface contamination,4,9 and prevalence among dental students and patients.10,11 Determination of the numbers and types of bacteria (including heterotrophic plate count bacteria [HPCs] and fecal indicators such as total coliforms and Escherichia coli) that occur on the variety of surface types present in the dental setting will provide useful information as to the general areas that might also serve as reservoirs for pathogenic microorganisms.12 Knowledge of the fomites commonly found in dental offices that are characterized by the highest levels of microbial contamination, coupled with the potential risks of pathogen transmission, can facilitate the development of a strategic surface-treatment hygienic intervention strategy employing the use of disinfectants.
Hydrogen peroxide (chemical formula: H2O2) is a naturally occurring oxidizing agent that produces short-lived free radical ions (HO- and HOO-). These ions can damage bacterial cell walls, thereby compromising cell wall integrity and resulting in an increased vulnerability to other disruptive agents (eg, detergents). The goal of this study was to determine the levels of HPCs, total coliforms, E. coli, and MRSA on various fomites in private practice dental offices, and to assess the impact of a hygienic intervention on the reduction of these bacteria following fomite disinfection using hydrogen peroxide-impregnated disposable wipes.
Materials and Methods
The 10 private practice dental offices selected for the study were located in Arizona (6) and Illinois (4). Each dentistry contained a minimum of two operatories, and two currently in use at the time of the study were selected for the sampling. A combined total of 285 fomite (surface) samples were collected prior to and following the surface disinfection wipe treatment between the hours of 12 p.m. to 3 p.m. on randomly selected patient care days (Monday through Thursday) over a 3-month period. A list of the surfaces evaluated and the number of samples collected per surface type appears in Table 1. In order to determine baseline prehygienic intervention enumeration data for HPCs, total coliforms, E. coli, and MRSA, approximately 4 square inches (41 cm2) of each fomite type were swabbed using a sterile Sponge-Stick infused with 10 mL of Letheen Neutralizing Broth by the manufacturer (3M, www.3m.com). For the surface disinfection intervention, an area measuring the same dimensions (41 cm2) and directly adjacent to the preintervention swab area was then disinfected according to manufacturer’s instructions using Hydrogen Peroxide Cleaner Disinfectant Wipes (The Clorox Company, www.clorox.com), and held for the recommended 1-minute contact time. One disinfectant towelette was used per study fomite, and then disposed of promptly. Upon closure of the contact period, the disinfected area was swabbed to obtain a post-disinfection sample using a fresh, sterile Sponge-Stick infused with 10 mL of Letheen Neutralizing Broth.
For fomites measuring less than 8 in2 in surface area, half of the visible surface area was swabbed to obtain baseline bacterial levels. The remaining nonsampled half portion of the fomite was then disinfected by wiping as previously described. Following the 1-minute contact time, the disinfected area was swabbed to obtain the post-treatment sample using a fresh, sterile Sponge-Stick infused with 10 mL of Letheen Neutralizing Broth. After sample collection, the Letheen Broth from all control and test swabs was expressed and collected into sterile tubes (average collected volume = 3 mL). Serial dilutions (1:10) of the expressed liquid were performed using physiological saline (0.85% NaCl), and heterotrophic plate count assays were conducted by dilution (10-1 through 10-4) and plating in duplicate onto R2A agarose medium (Difco, www.bd.com) using the spread plate method. The agar plates were incubated at 30°C for 5 days. Bacterial colonies were enumerated to determine the number of colony-forming units (CFU), and values were mathematically normalized to report CFU per 100 cm2.
Total coliforms and E. coli were concurrently assayed using the Colilert-Quantitray system (IDEXX, www.idexx.com). The Colilert reagent was rehydrated using 99 mL of sterile water, followed by the addition of 1 mL of sample extract from the swabs. Upon thorough mixing and pouring of the contents into Quantitrays, each sample tray was sealed and incubated at 35ºC for 24 hours. The trays were scored by visualizing and counting the large and small wells that demonstrated the positive color-change signal indicating the presence of total coliforms. E. coli was detected by exposing each tray to UV light. The large and small wells exhibiting both the positive color-change signal and fluorescence were scored as positive for the bacterium. The number of positive-score wells each for both total coliforms and E. coli were then converted to units of Most Probable Number (MPN) using a software calculator provided by IDEXX. Randomly selected samples that tested positive for E. coli were confirmed using the API 20E Identification System (BioMerieux, www.biomerieux-usa.com).
Methicillin-resistant Staphylococcus aureus (MRSA) was assayed for using the spread plate technique on Tryptic Soy Agar (TSA) amended with 5% sheep blood, 10 mg/L colistin, and 15 mg/L naladixic acid. The agar plates were then incubated at 35°C for 24 to 48 hours. Colonies demonstrating β-hemolysis (ie, complete lysing of red blood cells in the zone surrounding the colony) were transferred onto standard unamended TSA plates using the streak isolation method, and incubated at 35°C for 24 to 48 hours. Successfully isolated β-hemolytic colonies were subjected to a series of tests to confirm traits characteristic of MRSA including Gram staining for G(+) cocci cluster morphology, positive assay results for catalase and coagulase production (both tube and slide for the latter), and antibiotic resistance in the presence of polymixin B. Those colonies presumed as MRSA were then struck onto Methicillin-Resistant Staphylococcus aureus (MRSA)-specific CHROMagar (Becton Dickinson, www.bd.com), a selective and differential medium, for confirmation.
Results
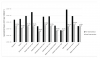
The geometric means and range values of HPCs for each fomite type sampled both pre- and posthygienic intervention are shown in Table 2. The average number of HPCs per 100 cm2 ranged from <3.00E1 (ie, 30) to 1.30E6. The greatest numbers were found on the office phones and the patient chair arm rests. The lowest levels were recovered from the dentist examination light. The geometric average of HPCs recovered from all sites sampled was <2.91E3, as several fomites, including the dentist chair arm rests, dental tool handles, cabinet handles, dentist examination lights, and patient room sink faucets yielded HPC bacterial levels below detection during the prehygienic intervention swabbing phase. Following treatment of the fomites with the disinfecting wipe, heterotrophic plate count bacteria levels were reduced by the disinfectant wipes overall to a geometric average of <1.60E2, with a more extensive list of fomites yielding bacterial levels below the limit of detection (30 CFU). The HPC levels measured prior to the intervention were statistically different (P = .0002) compared to the counts observed post-disinfection as determined by the Student’s t-test (P < .05). The patient chair arm rests and the office phones, which exhibited the highest preintervention HPC levels observed in the study, also saw reductions of >99% when calculated on a per-fomite-type basis (Figure 1).
In Table 3, the ranges of total coliform counts that were measured prior to and following the disinfectant wipe intervention are listed according to the various fomites sampled in the dental offices. The preintervention occurrence of total coliform bacteria ranged from none detected (<3 CFU per 100cm2) on the dental tool handles and dentist examination light, to greater than the maximum detectable level of 2.42E3 on the arm rests of both the patient and dentist chairs. No coliforms were detected on any of the fomites tested subsequent to the disinfectant wipe intervention. Similar to the coliforms, E. coli was most frequently detected on the patient chair arm rests (70%) and dentist chair arm rests (76%) as shown in Table 4. It was also detected on 25% of all bathroom doorknob and receptionist countertop samples, as well as on the computer mouse (7%) and computer keyboards (4%). Dental tool handles, patient room cabinet handles, dentist examination lights, office phones, office door knobs and patient room sink faucets yielded no detectable E. coli either before or after use of the hydrogen peroxide disinfectant wipes. Overall, and for all surface types evaluated, both total coliforms and E. coli were reduced by the disinfectant wipes >99%. Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from one office located in Illinois and from one in Arizona on a total of eight fomites prior to disinfection: one each of a dentist chair arm rest, doorknob, keyboard, office phone, in addition to two computer mouse devices and two receptionist counters. No MRSA was isolated following the hygienic intervention disinfectant wipe treatment of these surfaces.
Discussion
Bacteria were found in large numbers on fomites located throughout most of the common areas shared by patients and staff in dental offices. These findings reflect a lack of (or inadequate) disinfection in these high traffic areas. Cleaning alone is merely designed to remove dirt and grime from surfaces, and can actually facilitate the spread of bacteria and viruses throughout a facility; therefore, the use of disinfectants is key in preventing exposure to pathogens. Guidelines have been suggested for suitable levels of total bacterial numbers in the healthcare setting,13 with levels ranging from 250 to 500 CFU per 100 square-cm.14 These values were exceeded at least once on all fomite types sampled during the current study, with the exception of the dental tool handles and the dentist examination light. These guidelines do not reflect risk of infection, but serve as baseline recommendations to assess the effectiveness of disinfection and cleaning practices. Relative to preintervention bacterial levels, the use of disinfecting wipes decreased heterotrophic plate count bacterial numbers significantly (P = .0002). Following the hydrogen peroxide disinfectant wipe hygienic intervention, none of the geometric mean levels calculated for the study fomites tested exceeded 500 CFU cm2, although two surface types demonstrated mean values greater than 250 CFU cm2 (receptionist countertops and office doorknobs). Total coliform bacteria were found most frequently on the arm rests of both the dentists’ and the patients’ chairs (Table 3). Although coliforms are commonly found in feces, they may originate from other environmental sources such as certain foods. While their survival time is limited on dry surfaces, they can multiply on cleaning materials such as cotton cloths and sponges, thereby contaminating surfaces during the course of their use.15
The E. coli findings for the study were similar to those observed for total coliforms in that levels of the former were greatest on the high-frequency hand contact areas of the dentist and patient armchairs. Patients often grip the handles of the armchair during examinations, likely leading to the increased numbers detected on this type of fomite. The presence of E. coli indicates the presence of feces, although they may also grow in cleaning materials (eg, sponges) and be transferred during the wiping of surfaces.15 E. coli also tends to become inactivated on dry surfaces fairly rapidly; therefore, their ability to be readily cultured from fomites likely indicates recent contamination events.
Hands can be inoculated with coliform and E. coli bacteria by touching surfaces previously contaminated by other individuals, and then remain contaminated following improper washing during restroom use. In addition, cross-contamination of surfaces may occur when the sponges and cloth towels used to wash areas soiled with these bacteria are then subsequently employed to clean other materials. In a previous study, our research group demonstrated that the proper use of disinfectant cleaners in the home significantly reduces the occurrence of enteric bacteria.16 More recently, we reported even greater antimicrobial efficacy using disposable disinfecting wipes.17 The enhanced levels of microbial inactivation using disinfectant towelettes may be attributed to several factors, including the excess liquid that tends to be released by the saturated wipes when used, the willingness of individual users to allow this liquid to dry upon the treated surfaces after wiping (thereby increasing the antimicrobial contact time), and the wiping action itself, which also serves as a mechanism of removal (although the reductions due to wiping alone were not conducted in the current study). As a disinfectant active, hydrogen peroxide is highly oxidative and able to inactivate a broad spectrum of microorganisms including environmentally resistant bacterial spores such as Clostridium difficile, as well as enveloped and nonenveloped viruses.18 This study has demonstrated that the use of disposable hydrogen peroxide towelettes was effective in reducing HPC levels by >90% for most of the fomites evaluated (Figure 1), while total coliforms, E. coli, and MRSA were reduced to undetectable levels.
Although bacteria were found in high numbers prior to the disinfectant wipe hygienic intervention, these levels were likely due to the inadequate disinfection of fomites located throughout the common areas shared by patients and staff. The dental offices sampled in the current study reported adherence to the Centers for Disease Control (CDC) dental healthcare settings guidelines,19 which require sterilization procedures for patient care items, including instruments and devices depending on their categorization as “critical, semicritical, or noncritical.” The CDC also recommends the use of an EPA-registered hospital disinfectant for use on clinical contact surfaces (eg, light handles, faucet handles, and countertops) and housekeeping surfaces (eg, floors, walls, and sinks) when contamination is apparent. The dental offices participating in the study specified the use of quaternary ammonium compound-based disinfectant products (QACs) as recommended for the operatories and bathrooms, and a specialized QAC-alcohol formulation for oral patient-care cleaning tools. While many commercially available QACs are designated as broad-spectrum antibacterial agents, their efficacy depends largely on the directions for use indicated by the manufacturer with regard to maintenance of a wetted surface over the course a specified contact time. Nonadherence by dentistry or cleaning personnel to the label directions may have resulted in low levels of microbial reduction on office and operatory fomites. In addition, none of the dental offices surveyed reported regular wiping/cleaning of high-touch fomites including door handles and computer keyboards, with such cleanings conducted on an “as-needed” basis. Use of the same towels for cleaning a variety of surfaces throughout different areas of the dentistry, even when conducted inconsistently, may also facilitate cross-contamination via the spread of bacteria and other microorganisms. Therefore, the proper use of disinfectants and cleaning procedures is key in preventing the potential exposure of individuals to pathogens.
Fomites commonly located in the dental office and operatory settings may serve as reservoirs for a variety of bacteria including HPCs, total coliforms, E. coli, and pathogens such as MRSA. These bacteria may be transferred from fomite to fomite by human touch, and during cleaning procedures when sponges or rags are used to treat multiple surface types. In order to ensure a hygienic environment that is safe for patients, operatory staff, and office personnel, levels of bacteria and other microorganisms on surfaces should be minimized by effective cleaning and disinfection practices. The use of a broad-spectrum antimicrobial disinfectant towelette, when used according to the manufacturer’s instructions, can effectively reduce levels of common environmental bacteria and pathogens present on inanimate surfaces in the dental environment.
Conclusion
The present study examined the effects of a hygienic intervention employing disposable hydrogen peroxide infused towelettes for the disinfection of fomites commonly found in dental offices. The results reveal a statistically significant reduction in HPC levels, as well as a decrease in fecal-associated bacteria and MRSA to levels below the limit of detection on the evaluated fomite surfaces following the disinfectant wipe treatment. It is uncertain what percentage of the reduction was attributed to the mechanical action of wiping alone compared to the bactericidal action of the hydrogen peroxide active ingredient. Therefore, additional research may be warranted in order to further delineate the sources of efficacy demonstrated by broad-spectrum antimicrobial wipes that may be used by dental office personnel for fomite decontamination.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Sherri Carlino and Mr. Raj Lodhia for assistance in the conduct of this project.
DISCLAIMERS/DISCLOSURES
This study was supported in part by a grant to The University of Arizona from The Clorox Company.
Funding Sources for the Project: The Clorox Company
ABOUT THE AUTHORS
Charles P. Gerba, PhD, is a Professor at the Department of Soil, Water and Environmental Science at The University of Arizona. Gerardo U. Lopez, PhD, is an Assistant Professor at the School of Animal and Comparative Biomedical Sciences at The University of Arizona. Luisa A. Ikner, PhD, is a Postdoctoral Research Associate in the Department of Soil, Water and Environmental Science at The University of Arizona.
REFERENCES
1. Gerba CP, Pepper IL. Domestic and indoor microbiology. In: Environmental Microbiology. 3rd ed. New York, NY: John Wiley Publishing; 2015:665-675.
2. Lopez GU, Gerba CP, Tamimi AH, et al. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol. 2013;79(18):5728-5734.
3. Rusin P, Maxwell S, Gerba CP. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 2002;93(4):582-592.
4. Kurita H, Kurashina A, Honda T. Nosocomical transmission of methicillin-resistant Staphylococcus aureus via the surfaces of the dental operatory. Brit Dental J. 2006;201(5):297-300.
5. Bernardo WL, Boriollo MF, Goncalves RB, et al. Staphylococcus aureus ampicillin-resistant from the odontological clinic environment. Rev Inst Med Trop Sao Paolo. 2005;47(1):19-24.
6. Williams HN, Sigh R, Romberg E. Surface contamination in the dental operatory: a comparison over two decades. J Am Dent Assoc. 2003;134(3):325-330.
7. Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(S2):50-54.
8. Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: a review. Intl Dent J. 2001;51:39-44.
9. Lopes Motta RH, Groppo FC, Cassia Bergamaschi C, et al. Isolation and antimicrobial resistance of Staphylococcus aureus isolates in a dental clinical environment. Infect Cont Hosp Epidem. 2007;28(2):185-190.
10. Cássia Negrini T, Duque C, Mascarenhas de Oliveira AC, et al. Staphylococcus aureus contamination in a pediatric dental clinic. J Clin Ped Dent. 2009;34(1):13-18.
11. Zimmerli M, Widmer AF, Dangel M, et al. Methicillin-resistant Staphylococcus aureus (MRSA) among dental patients: a problem for infection control in dentistry? Clin Oral Inves. 2009;13(4):369-373.
12. Reynolds KA, Watt PM, Boone SA, Gerba CP. Occurrence of bacteria and biochemical markers on public surfaces. Int J Environ Health Res. 2005;15(3):225-234.
13. Lewis T, Griffith C, Gallo M, et al. A modified ATP benchmark for evaluating the cleaning of some hospital environmental surfaces. J Hospital Infect. 2008;69(2):156-163.
14. Dancer SJ. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hospital Infect. 2004;56(1):10-15.
15. Enriquez C, Enriquez-Gordillo ER, Kennedy DI, Gerba CP. Bacteriologic survey of used cellulose sponges and cotton dishcloths from domestic kitchens. Dairy, Food Environ Sanitation. 1997;17(1):20-24.
16. Rusin P, Orosz-Coughlin P, Gerba CP. Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. J Appl Microbiol. 1998;85(5):819-828.
17. Gerba, CP, Maxwell S, Sifuentes LY, et al. Impact of a disinfecting wipe on bacterial contamination in households. Household Person Care. 2013;8(3):24-26.
18. Linley E, Denyer SP, McDonnell G, et al. Use of hydrogen peroxide as a biocide: new considerations of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67(7):1589-1596.
19. Kohn WG, Collins A, Cleveland JL, et al. Guidelines for infection control in dental healthcare settings. Morbidity and mortality weekly report: MMWR. 2003; 52(RR17):1-61. Center for Disease Control.