You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Research and development of blocking anesthesia harkens back to 1784, when pressure-point anesthesia was used to compress nerves and vasculature to numb parts of the body. Cocaine was introduced 100 years later as the first local anesthetic for surgical procedures for glaucoma, followed in 1905 by procaine (ie, Novocain), which changed the way dentistry is performed. Despite procaine’s shortcomings (eg, high level of allergenicity, slow onset, short pulpal anesthesia duration), its use continued until the more potent and less allergenic lidocaine was synthesized in 1943. Demonstrating a more rapid onset, lidocaine remains safe and efficacious among the many local anesthetics available today.
The efficacy and safety of local anesthetics are dependent upon their molecular structure. How these molecules interact with the body—which vary among local anesthetics—impacts their potency and duration of anesthetic effects. Therefore, it behooves clinicians to understand the basics of local anesthetics, their componentry, and their mechanism of action in order to deliver them appropriately and effectively.
Basic Principles of Local Anesthetics
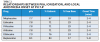
Local anesthetics are either esters or amides based on the aromatic ring in their molecular structure (Table 1). Other portions of their molecules include an amine group and an intermediate linkage. The aromatic portion is responsible for lipophilicity (ie, lipid/water distribution and protein-binding characteristics). The amine group, usually a secondary or tertiary amine, is associated with water solubility and carrying the molecule through the system, but is not necessary for anesthetic activity. Compounds lacking the amine portion are insoluble in water and useful only topically. The intermediate linkage, which connects to aromatic residue via an ester or amide linkage, determines the route of metabolism, allergic potential, and how the compound performs clinically.
The mechanism of action of local anesthetics involves blocking the sensation of pain by interfering with the propagation of nerve impulses along peripheral nerve fibers.1 This is accomplished by reducing the permeability of the nerve cell membrane to sodium ions and, with that decrease in sodium ions or closing down those sodium pumps, decreasing the rate of rise in the depolarization phase of the action potential, to the point that a propagated action potential fails to develop (ie, the nerve cannot send a pain signal because it has been blocked). Factors influencing local anesthetic effectiveness include its pKa, lipid solubility, and protein binding.
All local anesthetics are weak bases with a pKa range of 7.7 to 8.9. The pKa (ie, the free base or uncharged state that readily penetrates connective tissue and membranes) is responsible for the onset of anesthesia and how available in the system it will be (Table 2).1
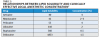
Lipid solubility affects potency, and the more lipid-soluble a local anesthetic is, the more potent it will be.2 Because nerve fibers are fat-rich cells, a higher level of lipid solubility enhances the potency of local anesthetics by ensuring the delivery of the anesthetic into the system. Local anesthetics that demonstrate a lower lipid solubility are typically provided by manufacturers in a higher concentration (Table 3).
Protein binding affects duration of anesthetic effects.3 Increased protein binding allows local anesthetic molecules to be more firmly attached to the proteins at receptor sites. The more tightly bound the local anesthetic is to the sodium channel and blocking it, the longer the nerve will be blocked and the longer the duration of action (Table 4).2,3
In addition to the affinity of the local anesthetic for the nerve membrane (ie, lipid and protein components), other factors affect the duration and efficacy of local anesthetics. These include type of injection/delivery, presence or absence of a vasoconstrictor, and whether the procedure involves pulpal or soft-tissue anesthesia.2 Although most local anesthetics are provided with a vasoconstrictor, some plain solutions remain available (Table 5). Local anesthetic selection will, therefore, be dependent upon multiple factors, including type of clinical procedure performed and level of anesthetic required and for how long. The elimination half-life of local anesthetics (ie, time required for metabolic breakdown and total elimination) will also be significant (Table 6), particularly in the context of managing local anesthesia overdose.4,5
Local Anesthesia Overdose
Symptoms of low-to-moderate overdose include apprehension, some confusion, and slurred speech (beyond that caused by numbness), in addition to an elevated heart rate, blood pressure, and respiratory rate. Signs of moderate-to-severe overdose include seizures in conjunction with central nervous system depression and depressed vital signs (ie, heart rate, blood pressure, respiration) (Figure 1).
When a patient exhibits symptoms of over-dose, treatment should be stopped, and the patient reassured and given oxygen via a controlled airway. Vital signs should be monitored, and someone should remain with the patient at all times. The patient should be closely monitored for the time necessary for the local anesthetic to distribute and be metabolized (ie, elimination half-life) based on the number of cartridges used; this, therefore, makes understanding the elimination half-life significant.4 Most local anesthetics have an average elimination half-life of 1.8 hours per 0.18-mg cartridge.
It is always advisable to remember that children, the elderly, and medically complex patients are more susceptible to local anesthetic overdose. It is also important to know the maximum doses of both local anesthetics (Table 7) and vasoconstrictors (Table 8).4
A good rule to follow is the rule of 25; use only one cartridge of local anesthetic for every 25 pounds of a patient’s weight. For example, six cartridges can be used for a 150-pound healthy male. This rule accounts for various anesthetic concentrations, as well as vasoconstrictor concentrations.
Articaine
Among the local anesthetics available to dentistry is articaine (eg, Septocaine®, Ultracaine®, Septanest®), which is supplied as 4% solution with 1:100,000 epinephrine and 1:200,000 epinephrine. First approved by the Food and Drug Administration in 2000, notable differences between articaine and other local anesthetics include a thiophene ring instead of a benzene ring, which improves diffusion through soft tissue and bone; an amide linkage that decreases the incidence of sensitivity reactions; and an additional ester linkage that increases metabolism by plasma carboxyesterases.
Despite the presence of a sulfur molecule, articaine is safe for patients stating they are sulfur sensitive; the sulfur contained in articaine is elemental, and allergic reactions to it are not possible. However, patients who are sensitive to sulfites—which are present in vasoconstrictors as preservatives—should receive a plain solution.
The elimination half-life of articaine is 1.8 hours, and the plasma half-life is 27 minutes. The duration of action is 1 to 4 hours, and the maximum dose is seven carpules; the rule of 25 still applies.6,7 Articaine’s onset time, duration, and anesthetic profundity are comparable to 2% lidocaine with 1:100,000 epinephrine (Table 9).6-8 However, the choice of local anesthetic remains up to the dentist based on a risk-benefit analysis per patient.
Many dentists use articaine based on growing clinical evidence supporting its efficacy. In one study examining infiltration injections in the mandible from second molar to first premolar, patients experiencing anesthetic success ranged from 75% in the second molar to 92% in the second premolar.9 This may be attributed to articaine’s molecular structure and diffusion through hard tissue. Another study reported similar findings in terms of pulpal anesthetic after patients received articaine, whether with 1:100,000 epinephrine or 1:200,000 epinephrine.10
This research confirms what clinicians have experienced clinically: that articaine is safe and effective when it is used in the right situations, on the right patients, and in the right circumstances with the proper injection techniques.
Paresthesia
Interestingly, despite their safety and efficacy, local anesthetics have been associated with paresthesia following nonsurgical (ie, not involving exodontia surgical procedures or implant placement) restorative dentistry. One of the more general groupings of nerve disorders known as neuropathies, paresthesia is an abnormal sensation that may manifest as total loss of sensation (ie, anesthesia), burning or tingling feelings (ie, dysesthesia), pain in response to a normally non-noxious stimulus (ie, allodynia), or increased pain in response to all stimuli (ie, hyperesthesia).11 Although the incidence of paresthesia is not truly known (ie, studies between 1998 and 2010 have cited ranges between 1:42 patients and 1:4.1 million patients12,13), theories to explain the causes of local anesthetic-induced paresthesia focus on direct trauma from the needle injection, hematoma formation, neurotoxicity, and inflammation. The latter three causes are generated from within the nerve.
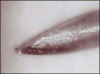
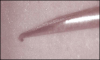
Direct trauma could result from hitting, nicking, cutting, or otherwise damaging a nerve during injection, particularly if blunt techniques involving bumping the bone and then drawing back are used.14 Bumping the bone creates a bur as the needle is drawn back (Figure 2 and Figure 3), which could traumatize the nerve. Additionally, the diameter of the needles used (eg, 25-ga: 0.508 mm; 27-ga: 0.406 mm; 30-ga: 0.333 mm) approximates one-fourth the size of the lingual nerve (eg, 1.86 mm); thus, it is understandable why lingual nerve paresthesia could result, and, in fact, this is where most paresthesia occurs.15,16
One explanation for most paresthesia occurring in the lingual nerve is that 33% of it is unifascicular (ie, one fiber).16 Whereas the inferior alveolar nerve is multifascicular (ie, as many as 25 or 30 fibers) and could continue functioning if one fiber were injured, the lingual nerve has a limited number of fascicles and could be traumatized by an injection needle.16
Closer examination of nerve anatomy shows that a number of layers (ie, epineurium, perineurium, and endoneurium) surround the nerve fibers, and each layer forms a protective sheath. This suggests that because the nerve is protected by so many levels, 4% anesthetic solution injected outside of and/or around the nerve is not likely a causative agent of paresthesia.15 Therefore, events that occur with injections inside the nerve have been implicated in paresthesia (eg, neurotoxicity, hematoma, neurogenic inflammation).17,18
Research examining the neurotoxicity of local anesthetics concluded that as the concentration of local anesthetic is increased, its neurotoxicity (ie, toxic effect on nerves) increases.17 All local anesthetics are neurotoxic, and their toxicity is concentration-dependent. Concentration-dependent toxicity causes apoptosis, which results from elevations of intracellular calcium.18 Among the more toxic local anesthetics are bupivacaines and tetracaines. The least toxic local anesthetics are articaines, prilocaines, and lidocaines.18
Although the causes of paresthesia are most likely multifactorial, intrafascicular injection appears to be required for damage (eg, trauma, neurotoxicity, edema, hematoma). Not surprisingly, to decrease the incidence of paresthesia, doctors are considering altering their injection technique rather than modifying the local anesthetic they are using.
Conclusion
It behooves clinicians to understand the basics of local anesthetics, their componentry, and mechanism of action in order to deliver them appropriately and effectively. Many brands of local anesthetics are available, and each can be safe and efficacious when used with the proper injection techniques, under the right circumstances, and for the appropriate patient. By applying knowledge of the significant considerations related to local anesthetics, clinicians can best ensure their patient’s safety while simultaneously delivering an excellent care experience.
ABOUT THE AUTHOR
Scott Dickinson, DMD, is owner of two Aspen Dental practices in Pensacola and Pace, Florida. He is an Army veteran and former flight surgeon, who graduated from the University of Pennsylvania School of Dental Medicine.
REFERENCES
1. Hersch EV. Local anesthetics. In: Fonseca RJ. Oral and Maxillofacial Surgery. Philadelphia, Pa: Saunders; 2000.
2. Jastak JT, Yagiela JA, Donaldson D. Local Anesthesia of the Oral Cavity. Philadelphia, Pa: Saunders; 1995.
3. Malamed SF. Handbook of Local Anesthesia. 3rd ed. St. Louis, Mo: Mosby Year Book; 1990.
4. Malamed SF. Handbook of Local Anesthesia. 4th ed. St. Louis, Mo: Mosby; 1997.
5. Wynn RL, Crossley HL, Meiller TF. Drug Information Handbook for Dentistry. 9th ed. Hudson, Ohio: Lexi-Comp; 2003.
6. Hawkins JM. Articaine: Truths, myths and potentials. Acad Dent Ther Stomat. ADA CERP. 2002:1-10.
7. Hawkins JM, Moore PA. Local anesthesia: advances in agents and techniques. Dent Clin North Am. 2002;46(4):719-732.
8. Feck AS, Goodchild JH. The use of anxiolytic medications to supplement local anesthesia in the anxious patient. Compend Contin Educ Dent. 2005;26(3):183-190.
9. Robertson D, Nusstein J, Reader A, et al. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138(8):1104-1112.
10. Abdulwahab M, Boynes S, Moore P, et al. The efficacy of six local anesthetic formulations used for posterior mandibular buccal infiltration anesthesia. J Am Dent Assoc. 2009;140(8):1018-1024.
11. Moore PA, Haas DA. Paresthesias in dentistry. Dent Clin North Am. 2010;54(4):715-730.
12. Food and Drug Administration. Septodont NDA 120-971. 1998.
13. Garisto GA, Gaffen AS, Lawrence HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010:141(7):836-844.
14. Almendros Marqués NA, Delgado Molina E, Tamarit Borrás M, et al. Comparison of two needle models in terms of bevel deformation during truncal block of the inferior alveolar nerve. Med Oral Patol Oral Cir Bucal. 2007;12(4):E317-E322.
15. Smith MH, Lung KE. Nerve injuries after dental injection: a review of the literature. J Can Dent Assoc. 2006;72(6):559-564.
16. Pogrel MA, Schmidt BL, Sambajon V, Jordan RC. Lingual nerve damage due to inferior alveolar nerve blocks: a possible explanation. J Am Dent Assoc. 2003; 134(2):195-199.
17. Hillerup S, Bakke M, Larsen JO, et al. Concentration-dependent neurotoxicity of articaine: an electrophysiological and stereological study of the rat sciatic nerve. Anesth Analg. 2011;112(6):1330-1338.
18. Werdehausen R, Fazeli S, Braun S, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103(5):711-718.