You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
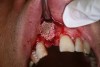
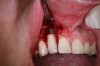
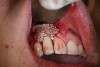
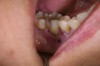
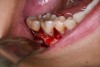
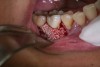
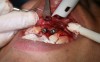
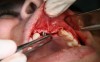
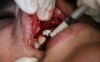
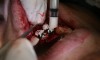
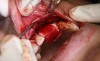
In practice, dentists may encounter a variety of bone defects, including extraction site defects (Figure 1 and Figure 2), fenestrations/dehiscence defects, horizontal ridge deficiencies (Figure 3 and Figure 4), vertical ridge deficiencies, and periodontal defects (Figure 5 through Figure 7).1,2 The most common, however, are extraction site defects that may compromise subsequent implant placement or stability of the ridge when no implant is planned.1,2
Following extraction, hematoma and clot formation occurs, along with granulation tissue formation. Blood clot formation is necessary at the wound site. Primary healing then begins. Granulation tissue is eventually replaced by connective tissue, which is subsequently supplanted by fibrillar coarse bone. Immature fibrillary coarse bone eventually generates into mature bone. Bone formation can be seen as early as 10 days.3
Unfortunately, however, whenever a tooth is extracted, bone resorption of the buccal and lingual walls, as well as at the alveolar crest, occurs. The extent of resorption is dependent on how dense the bone is as well as localized factors such as inflammation and infection. Buccal bone, being less dense then lingual bone, tends to resorb faster and to a greater degree. Regeneration is inhibited when the extraction socket is left unfilled at the time of extraction, and following site healing the socket suffers geometric depression of the buccal wall and some ridge resorption in both the vertical and horizontal dimensions. Additionally, soft tissue grows at a faster rate than bone and can invade the area of the bony defect, limiting full osseous regeneration.1,2
Without bone grafting and proper extraction site management, between 25% to 30% vertical bone loss may occur following extraction, along with 1 mm bone resorption and 2 mm gingival recession. Horizontally, 30% of buccal bone may be lost (ie, 2 mm to 2.5 mm), and 10% may be lost from the lingual. This may result in insufficient bone volume for placing an implant or to support non-implant fixed (ie, pontic) or removable prosthetics.1,2
Extraction site defects can be categorized into one of three classes1:
• Class I. Sockets with intact walls favorable for immediate implant placement with or without bone grafting.
• Class II. Sockets with missing labial/buccal wall, necessitating use of guided bone regeneration procedures in conjunction with implant placement.
• Class III. Sockets that do not provide implant anchorage and require staged implant insertion as well as bone grafting procedures.
Managing and Preserving Extraction Sites
Extraction site defects can be repaired by bone grafting the horizontal and/or vertical ridge deficiency. With grafting materials in place, bone fill is encouraged, and the ridge height and width can be better maintained. Research has shown that 79% of grafted sites had less than 20% buccal plate loss, compared to 71% of ungrafted sites that had greater than 20% buccal bone loss.2 Additionally, research has shown that the potential benefit of socket preservation therapies includes significantly less vertical and horizontal contraction of the alveolar bone crest.4
Socket grafting is the simplest, easiest, and most predictable technique for preserving the bone and soft tissue. Bone grafting material is placed in the socket after extraction and primary closure is achieved. Alternatively, a collagen plug is placed if primary closure is not achievable to help contain the graft material and provide a scaffold for the surrounding soft tissue to grow across.
Another technique for socket bone preservation is immediate implant placement, which is optimal for four-wall defects.1,4 Placement of the implant at the time of extraction helps support the socket walls and greatly limits atrophy as healing occurs. The implant is placed in the socket immediately after extraction, ideally engaging on the lingual portion where the bone is more dense. With a gap between the implant and socket wall on the buccal side, bone grafting material can be placed in that area. The buccal crestal bone is less likely to resorb during healing, because the gap ensures an absence of pressure on this delicate bone. It has been demonstrated that a gap of 2 mm or less does not require grafting and will fill on its own; this is related to the clot that will form following surgery. Gaps greater than 2 mm will require placement of a graft material to ensure filling of the gap following healing. A minimum bone thickness of 2 mm is required to surround the implant for stability.1,4
Guided bone regeneration can also be undertaken to manage defects in extraction sites where implant placement is planned. Guided bone regeneration is essential for two-to-three–wall defects and involves placing bone grafting material and/or the implant, along with a stabilized membrane.1,2,4
Biologic Requirements for Bone Grafting/Guided Bone Regeneration
Interaction among three basic biological elements is required for bone grafting and guided bone regeneration: cells (ie, for osteogenesis); growth and differentiation factors (ie, for osteoinduction); and extracellular matrix scaffolds (ie, for osteoconduction). Osteogenesis is the natural process of bone formation in the body and refers to the formation of new bone by the cells present within a graft or native site. The presence of viable cells (ie, osteoblasts) is the main rationale of osteogenesis and is required for bone generation.3 These cells are present in the patient’s circulatory system and arrive in the site via bleeding during surgery. They may also be added to grafting material (ie, platelet-rich fibrin [PRF], advanced-PRF, platelet-rich plasma [PRP]) derived from the patient’s blood to increase osteogenesis.
Osteoinduction is the process through which viable cells are recruited to the site and differentiated into bone-forming cells (eg, stem cell => osteoblast). The molecules (ie, proteins such as growth factors like bone morphogenetic protein [BMP]) contained within bone grafts facilitate this differentiation.3 Some allografts claim osteoinduction, and bone formation can be ectopi (out of place).
Osteoconduction is the physical process in which the graft acts as a 3D scaffold on which the cells are able to form new bone. Unlike osteoinduction, this is a passive mechanism, and osteoconductive properties are the minimum requirement for any bone grafting material.3
Ideally, the selected bone grafting material must demonstrate all three of these properties. However, because the objective in bone grafting is to grow bone in a defective area, the selected bone grafting materials must possess other requisite characteristics. They should be nontoxic, non-allergenic, and non-immunogenic, and promote healing without interfering with that process. They should be able to augment and grow bone in osseous defects and provide mechanical support when required. The material should be biocompatible (ie, bioinert to provide a passive scaffold) yet also bioactive (ie, to interact with tissue chemically to promote cell differentiation and proliferation).5,6 The goal is for the graft material to convert over a period of time into native bone in the area.
Among the materials available for bone grafting are autografts, allografts, xenografts, and alloplasts (Table 1).
Autografts. Autografts, or autogenous bone, are harvested directly from the patient from extraoral or intraoral sites. Intraoral sites for harvesting bone include the retromolar pad, maxillary tuberosity, mandibular symphysis, and ramus. Considered the gold standard because it demonstrates the fastest bone growth and all three essential properties (ie, osteogenesis, osteoinduction, and osteoconduction), autogenous bone is available in two types: cortical and cancellous. Despite their advantageous qualities, autografts do have disadvantages, including the need for a second surgical site and limited availability in the oral cavity, and they resorb quickly.5-7
Allografts. Allografts are bone grafts obtained from humans, usually cadaver bone that is available frozen (eg, freeze-dried and demineralized freeze-dried bone) or fresh. The age and health of the donor affects bone regeneration, and these bone graft materials require complex processing. Not surprisingly, disadvantages of allografts include risk of disease transmission, potential for immune rejection, inconsistent quality, and cultural acceptance by patients.5-8
Xenografts. Xenografts consist of bone derived from animals, most typically bovine, porcine, or equine. Like allografts, this bone material requires complex processing but does demonstrate osteoconductive properties. However, disadvantages to the use of xenografts include risk of disease transmission, potential for immune rejection, inflammatory response, cultural acceptance by patients, and an extremely slow resorption rate.5-8
Alloplasts. Alloplasts are synthetic bone graft materials that pose no risk of disease transmission or immunogenic response. Available in various shapes and sizes, alloplasts are osteoconductive and “radiodense,” so they demonstrate radiopaque properties.5-7 Although alloplasts are becoming more popular, they do produce slower cell turnover, and there is a perception issue regarding their use. Popular alloplast bone grafting materials include ceramic, beta-tricalcium phosphate (ie, TCP), and calcium phosphate silicate, among others.9,10
A new beta-tricalcium phosphate granule (PLGA) alloplast material (GUIDOR® easy-graft, Sunstar, www.guidor.com) was recently introduced that can be molded and shaped through the defect, where it can easily stay in place.11 After 2 minutes, the PLGA bone graft material, being a reverse thermoplastic (ie, becomes harder as it warms to body temperature), hardens into position without becoming compressed or deformed during patient movements, which helps to prevent bone/shape loss (Figure 8 through Figure 13).11 The benefit is the material acts as a 3D template to support the area being grafted as it is replaced by the patient’s own bone over time.
Extraction and Bone Fill Technique
The basic tenets of extraction and site preservation include atraumatic extraction, socket evaluation to ensure a clean area, adherence to grafting principles, and use of proven grafting techniques. A technique for using the aforementioned PLGA alloplastic bone material in the context of regenerating a socket defect is as follows:
(1) Atraumatically extract the tooth and debride the socket thoroughly using a piezosurgery, periotome, and the Benex® System (Benex, www.benex-dent.com). (2) Curettage and visually inspect the site to confirm the absence of periapical lesion or other soft tissue from the socket. (3) Sound and tap the site (ie, palpate/probe) to confirm the absence of buccal wall dehiscence or apical communication with other anatomy (ie, maxillary sinus or nasal fossa). (4) Take radiographs, as necessary, to ensure all walls are present and stable. This will help in determining whether preservation or regeneration should be undertaken. (5) Ensure that the periostea are intact, and that the surrounding soft tissue is preserved and demonstrates vascularity.
(6) Select a membrane to use to facilitate wound healing. The primary purpose of a membrane is to enhance would healing, as blood platelets are attracted to and aggregate on collagen molecules.12 When membranes are used, platelets release coagulation factors that work with plasma factors to initiate fibrin formation; this results in stable blood clots. Membranes also prevent mobilization of the grafting material, migration of soft tissue into the clot, and scar formation,12 and they limit gingival ingrowth into the grafting material as it organizes, allowing maximum osseous fill. Membranes are typically used in ridge augmentation procedures (eg, around implants, sinus lifts, multiple sites). When placing a membrane, ensure that it does not touch the adjacent tooth, leaving at least 1.5 mm to 2 mm of space.13,14
The two classifications of membranes are nonresorbable and resorbable.12 Nonresorbable membranes are mostly synthetic (eg, polytetrafluoroethylene [PTFE] or titanium) and, although they are biocompatible, they require re-entry for removal at a later time but can be left exposed. They are ideal for shape maintenance and when primary closure cannot be achieved. Resorbable membranes are the most commonly used and, although they typically resorb in between 45 to 150 days depending on the membrane material, they cannot be left exposed and primary closure is essential to their success. Available in collagen-based or synthetic types (polylactic acid/polyglycolic acid [PLA/PGA]), resorbable membranes are biocompatible and used when primary closure can be achieved.12
(7) Cut the membrane into an “ice cream cone” shape, and place it into the extraction area (eg, buccal wall). (8) Fill the socket with the bone fill/bone grafting material, being sure not to densely pack it. The bone grafting material should be loosely packed to allow capillary growth into the area. (9) Position the upper portion of the membrane (eg, the “ice cream”) over the bone grafting material to hold it in place.
(10) Select the appropriate suture material. Similar to membranes, suture materials can be absorbable (eg, catgut, collagen, vicryl, PGA) or nonabsorbable (eg, silk, nylon, polypropylene, PTFE), composed of monofilament or multifilament, and either natural or synthetic. The ideal suture material will demonstrate high tensile strength, minimal tissue reaction or irritation, knot security (ie, does not loosen up easily), and easy handling. It also should cause no postoperative pain and help to control hemostasis while maintaining flap positioning.
(11) Perform a figure-eight or horizontal mattress suture to secure the membrane over the bone material. To prevent degradation of the membrane, contamination, blanching, and other negative sequelae, be sure not to create too much tension. From approximately 6 mm below the height of the ridge in non-mobile tissue, enter the suture from the buccal, exit from the lingual at the same level of entry and exit height, and then come back from the lingual into the buccal and knot on the buccal aspect (Figure 14).
Conclusion
When dentists understand the basic types of bone grafting procedures, their options for bone grafting materials, and the indications for each, they will be better prepared when presented with the variety of common bone defects that could compromise implant treatments. Bone grafting materials have been successful in assisting in the restoration and maintenance of bone height and width, and new materials and techniques are continually helping to simplify the protocol involved with their placement. Using a new alloplastic bone material may also be beneficial.
ABOUT THE AUTHOR
Mike M. Chen, DMD, DICOI
Private Practice, California Center for Implant Dentistry, San Jose, California
REFERENCES
1. Saadoun AP, Landsberg CJ. Treatment classifications and sequencing for postextraction implant therapy: a review. Pract Periodontics Aesthet Dent. 1997;9(8):933-941.
2. Simion M, Rocchietta I, Kim D, et al. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26(5):415-423.
3. D’Mello S, Atluri K, Geary SM, et al. Bone Regeneration Using Gene-Activated Matrices. AAPS J. 2016 Sep 21. [Epub ahead of print].
4. Huynh-Ba G, Pjetursson BE, Sanz M, et al. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res. 2010;21(1):37-42.
5. Miron RJ, Zhang Q, Sculean A, et al. Osteoinductive potential of 4 commonly employed bone grafts. Clin Oral Investig. 2016;20(8):2259-2265.
6. Larsson L, Decker AM, Nibali L, et al. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95(3):255-266.
7. Dolanmaz D, Esen A, Yıldırım G, İnan Ö. The use of autogeneous mandibular bone block grafts for reconstruction of alveolar defects. Ann Maxillofac Surg. 2015;5(1):71-76.
8. Fernández RF, Bucchi C, Navarro P, et al. Bone grafts utilized in dentistry: an analysis of patients’ preferences. BMC Med Ethics. 2015;16(1):71.
9. Troeltzsch M, Troeltzsch M, Kauffmann P, et al. Clinical efficacy of grafting materials in alveolar ridge augmentation: a systematic review. J Craniomaxillofac Surg. 2016 Aug 18. [Epub ahead of print].
10. Yeo AB, Ong MM. Principles and implications of site preservation for alveolar ridge development. Singapore Dent J. 2004;26(1):15-20.
11. Leventis MD, Fairbairn P, Kakar A, et al. Minimally invasive alveolar ridge preservation utilizing an in situ hardening β-tricalcium phosphate bone substitute: a multicenter case series. Int J Dent. 2016; 2016:5406736. Epub 2016 Apr 14.
12. Merli M, Moscatelli M, Mariotti G, et al. Bone level variation after vertical ridge augmentation: resorbable barriers versus titanium-reinforced barriers. A 6-year double-blind randomized clinical trial. Int J Oral Maxillofac Implants. 2014;29(4):905-913.
13. Wang HL, Carroll MJ. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001:32(7):504-515.
14. Stadeker WJ. Grafting with cortical bone pins (the Alabama graft). Inside Dentistry. 2009;5(4):56-61.