You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Anxiety and fear continue to fuel avoidance of necessary dental care. To help mitigate these issues, dentists may offer various types of analgesia/sedation: nitrous oxide/oxygen analgesia, minimal or moderate enteral (oral) sedation, intravenous (IV) moderate or deep sedation, or general anesthesia. Training and license dictate the depth of sedation that can be offered/intended, but the depth of sedation that is achieved can be unpredictable, which introduces an additional element of risk. Benzodiazepines, opioids, antihistamines, and hypnotics (among others) are administered orally or parenterally to achieve various levels of sedation, as categorized in the Table.1 As the depth of sedation and cardiopulmonary function both lie on a dose and patient-response continuum, some patients may not fit exactly into this scheme.
More importantly, movement between levels of sedation can be rapid and can occur at unexpected doses. As loss of airway tone and ventilatory insufficiency (decrease in rate and depth) are expected and earlier-occurring side effects of these drugs—especially when given at higher doses, faster rates, or impatient sequential dosing—airway management becomes the cornerstone of patient safety.2 Any deficiency in airway management during sedation becomes a high-stakes, time-urgent situation because hypoxia can result in irreversible injury and death within minutes.
As a result of the wide variability of patient response, achieving and maintaining an appropriate level of sedation can be challenging. Because of this, the risk of sedation often becomes greater than the risk of the procedure that it was meant to enable, especially when patients descend to a deeper than intended level of sedation and either drug reversal or interruption of the procedure to allow spontaneous recovery to a lighter level of sedation is not entertained. This risk can be managed.
To date, there is no central reporting agency or database in the United States that tracks the morbidity and mortality of sedation/anesthesia in the dental office. When sedation-related death does occur, it often feeds national attention, especially when blatantly compared to a “menial” task that it facilitated. Bennett et al3 recently estimated the frequency of brain injury or death as a result of deep sedation or general anesthesia in the dental office to most likely exceed 1 per month. Based on well-informed but still anecdotal reports, this estimate is not improving. A recent Dallas Morning News investigation reported a much higher estimate.4 Many of these adverse events are known to have been triggered by “practice deviations.” Regardless of an overall low frequency, these events are high impact and, as such, command immediate attention and sustainable, visible effort at the individual, team,5 state, and national levels to improve and prevent.
The problem is not unique to sedation in the dental office. About 15 years ago, the Institute of Medicine reported that 98,000 patients needlessly die each year because of preventable medical harm.6 Dr. Lucian Leape (the report’s co-author) opined that this number would be equivalent to several fully loaded jumbo jets crashing each week. Ten years later, Clancy et al7 reported that patient safety was actually getting worse, instead of better. In the early 1980s, the medical discipline of anesthesiology, singularly, began and has been successful with its efforts to reverse negative trends in patient outcomes first by identifying and then by remediating triggers of anesthetic catastrophe through the creation of a database, improving safety efforts (notably the addition of the third year of anesthesia residencies and the establishment of the Anesthesia Patient Safety Foundation, among others), improving monitoring (pulse oximetry, then capnography), and the adoption of systems and protocols to minimize the incidence and consequences of human and systems error, which can be improved but, unfortunately, not entirely eliminated.8-11
THE PROBLEM
Human (cognitive, individual-oriented skill) error12 is a factor in up to 70% of deviations from safe practice that result in patient injury during anesthesia care.8 Poor, inadequate, or faulty teamwork and communication (social, team-oriented skills) were also identified as contributing to patient harm.12-14
Deviations from safe practice often results from aberrant mental processes of an individual practitioner.11 As humans are fallible (inborn human weakness), many of these errors are expected, not surprising, and, in the opinion of some, must occur.15 Included here are forgetting, inattention, slips, carelessness, unfamiliarity with the circumstances (deficiencies in training or experience), fatigue, thought burden, substance abuse, performance decrement at extremes of arousal, habituation, complacency, and “practice drift” (the insidious invasion of unsafe practices). These errors are considered unintentional, vis-à-vis violations, which are deliberate attempts (errors) to achieve a goal that is incompatible with safe practice.11 Violations are often committed because of previously identified “errors of thought”: consistent irrationality and game theory, among others.
Consistent irrationality theories include sunk cost (an unwillingness to retreat from fruitless intervention in order to validate prior action) and prospect theory (a fear of reprisal or legal action invites the pursuit of futile efforts to avoid loss). A tenet of game theory regarding situational choices proposes that conservatism will prevail as risk increases; however, in clinical practice, the most conservative approach may not be the best, especially during airway compromise. Approaches to improve individual performance (reducing fallibility) include continuing education, simulation training and rehearsal, focusing on the task at hand, eliminating distractions, and maintaining a healthy, rested, nourished, and stress-minimized state, among others. Engagement in a culture of safety optimizes these efforts. Punishment, shame, legal action, and limitation of privileges or licensure seem to have little effect at improving inevitable human error and may even hamper the trust required for incident reporting.
Deviations from safe practice can also result from systemic or latent conditions11, which often times can lay dormant in a process for many years. Included here are inadequate patient screening/scheduling, understaffing, undertrained staff, lack of protocols, lack of equipment, and so forth. System deficiencies can be fixed by structuring error-resistant systems16 (“safety nets,” eg, checklists, flow sheets, continuing education and rehearsal, team training, etc.) to minimize errors as well as the resultant adversity that can but may not always occur after an error incident. Improving these “soft” or “nontechnical” skills occurs via crisis resource management (which is covered later in this article).
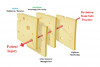
Similar to high-reliability organizations (eg, nuclear aircraft carriers, which carry airplanes and a nuclear power plant, etc.) the environment of sedative drug administration to patients is complex, uncertain, high-risk, and subject to rapid and unpredictable change. When coupled with unpreparedness (surprise) and time urgency, human and systems errors can readily accumulate, and, when temporally aligned, will result in adversity or “sentinel events”—unexpected, unplanned, unintended, and undesirable patient outcomes or injury. The now classic Swiss cheese model of accident theory11 provides a clear visual concept (Figure 1). Deviations from safe practice will result in patient injury only when the holes (human or systems errors) in each slice of Swiss cheese (defenses) have temporarily, temporally, and spatially lined up to allow penetration of the injury trajectory. Vigilance, monitoring, practitioner skill, team training, and “appropriate” patient selection represent the cheese slices (defenses) that serve to prevent deviations from causing injury. Unfortunately, this does not always happen in an imperfect practice environment.
THE PREMISE
“Only” sedation does not appear to improve safety.17-19 Deeply sedated patients can lose airway patency, just as patients under general anesthesia can. Most notably (and unlike the relaxed, paralyzed state of general anesthesia prior to endotracheal intubation), a deeply sedated patient can present with a mouth clenched closed and in an “unrelaxed” state, hampering the triple airway maneuver needed to open and gain visual access to the airway. Deeper than intended levels of sedation due to unintentional (absolute or relative) overdose can be unexpected and not readily noticed or appreciated. Resultant respiratory depression (a loss of airway patency and/or hypoventilation) triggers hypoxia, which may not be immediately recognized, accepted, or remediated. Bhananker et al17 has concluded that at least 50% of claims noted in their review of injury during monitored anesthesia care could have been prevented by better monitoring (including capnography), improved vigilance, and audible alarms. Human error, once again, has been shown to often be contributory.15
It is important to keep mindful of the fact that the “average” initial dose of any medication is determined from the normal distribution graph (a bell-shaped curve). On occasion, any given patient on any given day may be hyper-responsive to a “normal” weight-based dose. Unintentional overdose is more likely in patients at age and weight extremes, in patients with eating disorders (eg, anorexia, bulimia), obstructive sleep apnea, or substance abuse disorders, who are taking psychotropic medications, and who are temporarily hypovolemic from prolonged fasting or recent diuretic use, among others.
Loss of airway cannot always be predicted, but should always be anticipated. To reiterate, ability to maintain an airway can be lost in patients who are not fully asleep.
Less morbidity is expected with healthier patients when challenged with the same anesthetic insult, although this cannot be tested. Patients with normal lung volumes, especially when pre-oxygenated, can tolerate apnea for a longer period of time; patients with obesity, pulmonary disease (especially with recent exacerbation), cachexia, or young age cannot, as lung volumes can have proportionally less volume. Patients with coronary artery disease may be less able to tolerate the increased cardiac workload that accompanies hypertension or tachycardia, often triggered by hypoxemia or poorly conducted sedations.
Patients who are provided with supplemental oxygen prior to and during drug administration may be able to tolerate apnea without hypoxemia for a longer period of time, ostensibly providing the clinician with extra time (a margin of safety) to remediate the ventilatory problem. However, supplemental oxygen delays the onset of hypoxemia due to apnea, which temporarily blinds the clinician to apnea.
THE SOLUTION
There is no easily or readily attained solution to eliminate the risk and morbidity associated with sedation in the dental office. Safe, successful, and repeatable patient sedation can be achieved when the following six disciplines affecting patient care are optimized. They correlate with practitioner training, knowledge and ability, team training and competence, facility preparedness, anesthetic technique, and patient selection. These six “Patient Safety Initiatives” can be used to minimize the risk associated with office sedation and to provide a blueprint to improve safety:
• Establishing a culture of safety and a database of adverse events
• Improving patient selection
• Depth of anesthesia limit setting
• Improved monitoring
• Basic emergency airway management training and rehearsal
• Crisis resource management
1. Establishing a Culture of Safety and Database of Adverse Events
Safety culture is defined by NASA as an “environment where everyone works safely, feels comfortable communicating safety issues, learns from mistakes and successes, feels confident balancing challenges and risks while keeping safety in the forefront, and trust that safety is a priority.20 Safety is something that is actively and continually pursued, it does not “just happen.” It becomes more important than the goal. In any safety culture, there is a collective preoccupation with the possibility of failure; errors are welcome and held in open view as an opportunity to improve systems that limit or minimize adversity. Effort to sustain an effective safety culture comes from all entities: dentist, office team, legal, state and national agencies, and professional organizations.21 High-reliability organizations suffer less than their “fair share” of adversity,11 largely in part due to their pervasive safety culture. The medical specialty of anesthesiology has likewise markedly improved their outcomes in similar fashion.22 A culture of safety is both possible and effective.
Nonpunitive and voluntary reporting of close calls,2 morbidity, and mortality would be a first step to understanding the nature and frequency of adversity and improving effective risk management. In kind, this database would also guide the efficacy of proactive changes to improve safety. A degree of anonymity or the existence of an intermediary third party can help eliminate self-reporting biases and improve database accuracy. Patients, practitioners, staff, insurance companies, state agencies, professional organizations, and legal entities would be enticed to populate the data bank once an expansive and well-publicized culture of safety is embraced. Information needs to be interpreted carefully by a panel of experts.
2. Improved Patient Selection
Patient selection is, by far, the most complex part of the safety equation. At first glance, it would appear that a healthy, athletic adolescent with a large airway space would be at less anesthetic risk than an obese, elderly, hypertensive, diabetic man with obstructive sleep apnea and a long history of cigarette smoking. Indeed, this would be an easy decision. However, these healthy patients are still prone to airway compromise, aspiration, or pulmonary edema. When to “say no” becomes a more difficult question when patients present with less obvious or serious medical and anatomic challenges. The question is confounded by a complex interplay among practitioner knowledge, skill and ability, team training and functioning, anesthetic technique, level of monitoring, and facility “preparedness.”
Sedation in the dental office is often elective, and can be refused. Careful patient selection is initially guided by thorough evaluation: history and physical examination. Patients are often unaware of the presence or severity of diseases, either when undiagnosed, undisclosed, or forgotten. As such, any presence of risk factors for pulmonary or cardiac disease would, de facto, lead the practitioner to assume that disease is present: “Suspicion clinches diagnosis.” The cigarette smoker has chronic obstructive pulmonary disease, the obese patient often has diabetes and hypertension, the patient who snores has obstructive sleep apnea, any indication of a difficult airway should invite concern, and so forth.
Among the many adverse events that can occur during office-based sedation, there are two that stand out. The first is drug-induced hypoventilation and/or loss of upper airway tone, and the second is adverse cardiovascular changes. With the notable exception of ketamine and the notable implication of opioids, anesthetic medications (especially when used in synergistic combination, where individual drug action can be significantly intensified) can lead to a dose- and rate-of-administration–dependent loss of airway tone and hypoventilation/apnea, leading to hypoxemia, which initially triggers a sympathetic surge. Complex cardiovascular changes in heart rate and blood pressure can also occur secondary to both drug action and the “neuro-endocrine stress response” of a poorly conducted anesthetic—triggering sympathetic surge (tachycardia and hypertension). With this in mind, three parameters are then assessed to inform appropriate patient selection: airway, resilience, and reserve.
Regardless of health status, airway and ventilatory compromise can occur in all patients. Multiple airway parameters should be assessed to inform decisions about case acceptance or depth of anesthesia limit-setting. Mandatory airway assessment, including Mallampati scores, should be recorded and heeded. Like other airway assessment tools, when considered on an individual basis this score lacks both sensitivity and specificity; however, it entices the practitioner to perform and incorporate a complete and thorough airway examination. Classic airway assessment for sedation seeks, at a minimum, to predict the likelihood of the following three situations: (1) spontaneous airway collapse after drug administration; (2) the ability to open a collapsed airway with a chin lift/head tilt/jaw thrust maneuver; and (3) the ability to attain an airtight facemask seal to allow positive pressure ventilation to stent a potentially collapsed airway.
After inspection of the size and position of the mandible, the maximal opening, jaw protrusion, and neck extension (ie, ability to “sniff the morning air”) are assessed. Visual intraoral inspection notes the size and position of the tongue in the oropharynx. In like fashion, the size, tone, and mobility of the spaces surrounding the tongue should be evaluated—can either the tongue be displaced inferiorly or can the mandible be protruded to allow tongue displacement away from the posterior pharyngeal wall? Large tonsils or tongue encroach, while teeth and trismus (limited opening) obscure the airway space. Small or retrusive mandibles and narrow palates crowd the tongue, and an inability to protrude the mandible and/or extend the neck will limit the ability to improve airway patency or access. The ability to seal a facemask and provide positive pressure ventilation is hindered by age, jaw deformities, lack of teeth supporting the lips, facial hair, and a flat nasal bridge, among others.
Patient resilience is the physiologic ability to tolerate these insults. Apnea or the inability to ventilate is less tolerated when patients have not been pre-oxygenated or have diminished functional residual lung capacity, whether from age extremes, obesity, pulmonary injury, anorexia, cachexia, and skeletal malformations, among others. Hypotension is less tolerated in volume-depleted patients (eg, from prolonged fasting on a hot, humid day), in patients with underlying rhythm abnormalities, and in patients in the “beach chair” position, which can impede venous return and cardiac output. Hypertension and/or tachycardia both increase myocardial oxygen demand, a situation that should be avoided with coronary artery disease, which limits myocardial oxygenation.
Patient reserve is the physiologic ability to compensate for hypoxemia, which is hampered by a loss of airway or opioid-induced hypoventilation; or hypotension, when a compensatory tachycardia, increased contractility, or vasoconstriction is hampered by opioids or beta-blockers. The topic of resilience and reserve is greatly expanded elsewhere.23
Depth Of Anesthesia Limit Setting
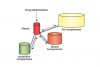
Intended drug action occurs when drugs diffuse to and bind to their receptor sites, eg, the vessel-rich central nervous system (CNS), to trigger responses such as anxiolysis, sedation, amnesia, analgesia, and/or hypnosis. Drug effect terminates once the drug diffuses away from its receptor site and undergoes metabolism or excretion. Regardless of the route of administration, drugs also accumulate in fat or muscle stores (where they do not exert clinical effect). When drugs leave fat or muscle stores and before metabolism or excretion, they can once again bind to CNS receptors to trigger a response (Figure 2). Hence, slow-onset drugs, or drugs that accumulate in fat or muscle stores (especially in patients with large fat or muscle stores) become difficult to titrate, especially when administered orally or intramuscularly. As such, impatient or repeated dosing can easily lead to overdose. A thorough understanding of pharmacokinetics combined with a “start low and go slow” technique minimizes the possibility of overdosing, and facilitates depth of anesthesia limiting-setting.
Drug response is also difficult to predict as the dose and rate of administration effects fall on a normal distribution curve, inviting the possibility of a diminished or exaggerated drug response. It is known, however, that patients with obstructive sleep apnea are more sensitive to opioid-induced respiratory depression,24 and that elderly patients requires a 50% to 75% initial dose reduction, taking no other parameters into account. Inadequate patient history (“did not ask or did not tell”), as well as concealed or denied substance abuse disorders also challenge the predictability of drug response.
Patients should be made aware of the depth of anesthesia limit-setting and accept the possibility of subjective “undersedation,” when the depth of anesthesia is limited by license, training/ability, troublesome airways, or patient resilience or reserve.
Improved Monitoring
Vigilance is the motto of the American Society of Anesthesiologists.25 It is defined as the action or state of keeping “careful” and continuous watch for possible danger or difficulty. Anticipating critical events makes their timely resolution more likely. Peri-anesthetic visual patient observation, intermittent blood pressure recording, pulse oximetry, and continuous electrocardiography are standard and well-accepted requirements for moderate and deeper levels of sedation. Recent recommendations for end-tidal capnography during moderate or deeper sedation have made.26 It remains difficult, if not impossible, to demonstrate that robust monitoring does not improve outcomes, given the fact that patients can quickly and unexpectedly move between levels of sedation. In fact, it may be risky to wait for such evidence.27 Because it is well accepted that a sedation provider should be able to rescue patients from one level deeper than intended, it is reasonable that the patient should be monitored as appropriate for one level deeper than intended sedation. Because a loss of airway tone/hypoventilation are expected side effects of most anesthetic agents, as variation in patient response to medication remains an ever-present possibility, and as most anesthetic morbidity in the dental office relates to hypoxemia, it seems plausible to monitor these events with utmost intensity. Pulse oximetry indirectly measures (in a delayed fashion of up to 30 seconds) the partial pressure of oxygen in the blood; capnography measures the depth, rate, and cadence of ventilation (and indirectly circulation) when exhaled carbon dioxide is captured (with a 6- to 8-second delay), and pre-tracheal auscultation immediately informs of changes in airway caliber and tone, based on the appearance of adventitious breath sounds. Monitoring is optimized when all three methods are coupled with continuous, visual patient inspection for chest movement and skin color by the anesthesia team. “It is ironic that more stringent standards of monitoring are not enforced when sedation is administered by non-anesthesiologists, some of whom may not be as adept as anesthesiologists in securing and maintaining ventilation.”28
Basic Emergency Airway Management Training and Rehearsal
Expected adverse effects of most anesthetic agents include a loss of upper airway patency (supraglottic obstruction) secondary to relaxation; inspiratory airway collapse; posterior displacement of the tongue, soft palate, and/or epiglottis; and hypoventilation or apnea. Any instance of even a 1% drop in SpO2 should be met with immediate full attention and assuredness that oxygen delivery to a patent airway is occurring.
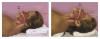
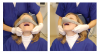
Basic skills for maintaining or recapturing (opening) a lost upper airway during sedation include noting and recording the time of obstruction, maintaining an open mouth, suctioning of the oropharynx, ensuring the delivery of 100% oxygen, and attempts to arouse the patient. This should occur concurrently with the triple-airway maneuver (Figure 3)—the sniffing the morning air position: flexion of the lower neck, extension of the upper neck, and protrusion or thrusting forward of the mandible. This manipulation is intended to pull the tongue, epiglottis, hyoid bone, and associated soft tissue forward, to open or increased the caliber of the oropharynx and hypopharynx. When these maneuvers fail, the extended head can be rotated approximately 30º to either side to facilitate airway opening (Figure 4). When this fails, a full facemask with self-inflating bag (BVM: bag, valve, mask) should be sealed over the mouth and nose. The bag is squeezed to force oxygen-enriched air into the airway, hopefully stenting the tongue and palate out of the way to allow oxygen entry into the lungs. Proper ventilation with a BVM is the “brass ring” of airway management, making it a skill that should be practiced often. Oropharyngeal or nasopharyngeal airways and supraglottic devices should be used when positive pressure ventilation cannot be achieved without their use. Drug reversal can be entertained to awaken an obstructed patient; however, this would not be an initial approach and should not delay the triple-airway maneuver and BVM ventilation.
It is most important that the entire sedation team understand and frequently rehearse during non-patient scheduled time their roles and responsibilities concerning the flow of events and movement of devices during these sequential airway management techniques. Assistants should be able to act without waiting for prompts or directives from other team members during these crucial minutes. Protocols (checklists) should be committed to memory and posted in highly visible locations. Several different styles of airway manikins are available for purchase to aid in simulation training in the office. Alternatively, local organizations can procure the manikins and make them available to staff members.
Crisis Resource Management
Crisis resource management is a series of techniques/actions/protocols for managing time-urgent, high-stakes events in the medical environment.9 These protocols seek to improve the social, team-oriented, “nontechnical” skills as a viable mechanism to prevent any deviations from safe practice from causing patient injury—making the environment (system) more “error resistant.” By optimizing and using all available resources (self, staff, monitored, airway devices, cognitive aids, colleagues, paramedics, educational opportunities, etc.) the ability to translate “knowing what to do” into effective performance is enhanced. The following are exemplary salient components of crisis resource management as it applies to sedation safety in the dental office before, during, and after patient care.2
Anticipate and Plan
Consistent with safety culture, practitioners should always plan for the worst. Emergency scenarios should be anticipated, and rescue plans consistent with the nature of the practice should be devised and continually rehearsed by all staff. This is called simulation,12 which is an enactment of something anticipated, meant to reinforce roles, duties, movement of various devices, and protocols for the management of rare emergency situations. Frequent rehearsal improves the retention of these tasks. Errors during simulation do not result in patient harm and can be noted to eliminate their recurrence. During simulation, all team members become aware of their duties and can perform them without waiting for prompts or others to act. The environment can be prepared for various emergencies. The three skill sets that are addressed are affective (interacting), cognitive (thinking), and psychomotor (doing). Affective and cognitive simulation requires participation of the entire team, best accomplished in the office environment (“in situ”). As each office is unique, individual nuances in diagnosis and treatment protocols can be incorporated. Psychomotor training—such as airway interventions, electrical therapy, and auscultation, etc.—are best practiced on a manikin; however, manikin proficiency does not guarantee human proficiency. The key principle here is: practice—repeatedly.
Know the Environment
Before commencing any patient treatment, a “time out” should be taken, where all important features of the case are announced out loud for all to hear—patient name, health concerns, status, procedure to be performed, etc. At this time, an equipment check is also done: monitors, back-up devices, connectors, gas sources, suction, etc. The plane is on the runway, so to speak, and ready for takeoff.
Use All Available Information
During any emergency, the team leader must perceive and process all pertinent information: vital signs, status of intravenous access, patient color, etc. Each team member should know individual responsibilities before any emergent situation arises. Quick glances or fast checks often leave the leader with misinformation.
Allocate Attention Wisely
Optimal situational awareness keeps the practitioner informed of the entire environment, while attending to a single problem. A repeating cycle of perceive–process–perform–perceive–process–perform facilitates movement through a situation. Priorities can be set and changed as needed, re-evaluation is frequent, and fixation errors minimized. During an airway crisis, the first intervention is frequently but sometimes erroneously presumed to be the correct intervention, when it many cases, it is not.
Mobilize Resources
These resources can be additional personnel (colleagues, key staff members), additional monitors, airway devices, or video laryngoscopes. In any event, at least one back-up airway plan should be formulated and immediately available.
Use Cognitive Aids
Performance decrement during stressful, high-stakes, time-urgent situations occurs almost universally. Mental and sometimes physical “situational paralysis” can occur, grossly interfering with thought processes. During these times, checklists can jog memory and guide direction for crisis resolution. Checklists should be posted in visible locations for all to see.
Communicate Effectively
During crises, demands or requests are often shouted out, but no one hears. Closed-loop directed communication is most effective, eg, “Mary, please check patient saturation.” “Yes, doctor, I will check saturation, it is …..” Junior team members should not be hesitant to speak up, as authority grids flatten when all intentions are focused on patient safety.
Distribute the Workload
The practitioner will need help in urgent times, and will not have the time or inclination to start assigning tasks in the moment of crisis. Staffing should be adequate, informed, trained, and patient scheduling should be unhurried.
Call for Help Early
There is no shame in calling for help, such as colleagues or activation of the local EMS. It is always better to be safe than sorry, and this action should be praised. It does not indicate a lack of qualification.
CONCLUSION
The pursuit of a culture of safety as a tool for risk management is enhanced when it is embraced and well publicized by all: the individual practitioner; the office staff; local, state, and national organizations; insurance companies; and legal entities. The time for improvement is now.
DISCLOSURE
Dr. Bosack received an honorarium from DENTSPLY for the preparation of this manuscript.
ABOUT THE AUTHOR
Dr. Bosack received his DDS from Loyola University School of Dentistry and completed a residency in oral and maxillofacial surgery at Cook County Hospital. He is active in private practice and also serves as a clinical assistant professor at the University of Illinois College of Dentistry. He is editor and author of Anesthesia Complications in the Dental Office (2015, Wiley Blackwell). He is the originator, developer, and executive director of Dental Anesthesia Online (www.daoce.org), an online continuing education resource in anesthesia. He currently serves on the Examination Committee for the American Board of Oral and Maxillofacial Surgery and has been named a content expert in anesthesia. He is a frequently invited speaker at state, regional, and national meetings. He has authored/coauthored numerous publications related to anesthesia.
REFERENCES
1. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-anesthesiologists. Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology. 2002;96(4):1004-1017.
2. Rall M, Dieckmann, P. Safety culture and crisis resource management in airway management:
General principles to enhance patient safety in critical airway situations. Best Pract Res Clin Anaesthesiol. 2005;19(4):539-557.
3. Bennett JD, Kramer KJ, Bosack RC. How safe is deep sedation or general anesthesia while providing dental care? J Am Dent Assoc. 2015;146(9):705-708.
4. Egerton B. Deadly Dentistry–a Dallas Morning News Investigation. December 9, 2015. http://interactives.dallasnews.com/2015/deadly-dentistry/index.html. Accessed Dec 13, 2015.
5. Treasure TE. Beware of “black swans” and “perfect storms:” the principle of plenitude and office-based anesthesia. J Oral Maxillofac Surg. 2014;72(8):1441-1443.
6. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Institute of Medicine Committee on Quality of Health Care in America. Washington, DC: National Academies Press (US); 1999.
7. Clancy CM, Munier W, Crosson K, et al. 2008 National Healthcare Quality Report. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/research/findings/nhqrdr/nhqr08/index.html. Accessed December 3, 2015.
8. Cooper JB, Newbower RS, Kitz RJ. An analysis of major errors and equipment failures in anesthesia management: Considerations for prevention and detection. Anesthesiology. 1984;60(1):34-42.
9. Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63(9):763-770.
10. Gaba D, Fish KJ, Howard SK. Crisis Management in Anesthesiology. Philadelphia, PA: Churchill Livingstone, 1994.
11. Reason J. Human error: models and management. Brit Med J. 2000;320(3):768-770.
12. Ostergaard D, Dieckmann P, Lippert A. Simulation and CRM. Best Pract Res Clin Anaesthesiol. 2011;25(2):239-249.
13. Haller G, Laroche T, Clergue F. Morbidity in anaesthesia: today and tomorrow. Best Pract Res Clin Anaesthesiol. 2011;25(2):123-132.
14. Murray AW, Beaman ST, Kampik CW, Quinlan JJ. Simulation in the operating room. Best Pract Res Clin Anaesthesiol. 2015;29(1):41-50.
15. McIlvaine WB. Human error and its impact on anesthesiology. J Crit Care. 2006;25(3):172-179.
16. Ruskin KJ. Safety and Human Factors in the Operating Room. American Society of Anesthesiologists. Refresher Course Lectures, 2012.
17. Bhananker SM, Posner KL, Cheney FW, et al. Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology. 2006;104(2):228-234.
18. Cote CJ, Notterman DA, Karl HW, et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105(4 Pt 1):805-814.
19. Cote CJ, Karl HW, Notterman DA, et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106(4):633-644.
20. Office of Safety and Mission Assurance. Safety Culture. 2015. https://sma.nasa.gov/sma-disciplines/safety-culture. Accessed Dec 3, 2015.
21. Arfanis K, Fioratou E, Smith A. Safety culture in anaesthesiology: Basic concepts and practical application. Best Pract Res Clin Anaesthesiol. 2011;25(2)229-238.
22. Botney R. Improving patient safety in anesthesia: a success story? Int J Radiat Oncol Biol Phys. 2008;71(1 Suppl):S182-S186.
23. Bosack RC, Lieblich S, eds. Anesthesia Complications in the Dental Office. 2015; Wiley-Blackwell.
24. Mickelson SA. Preoperative and postoperative management of obstructive sleep apnea patients. Otolaryngol Clin North Am. 2007;40(4):877-889.
25. Bacon DR. Iconography in anesthesiology. The importance of society seals in the 1920s and 30s. Anesthesiology. 1996;85(2):414-419.
26. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. October 2015. http://www.asahq.org/~/media/sites/asahq/files/public/resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf. Accessed Dec 3, 2015.
27. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501-507.
28. Kodali BS. Capnography outside the operating rooms. Anesthesiology. 2013;118(1):192-201.