You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
INTRODUCTION
Multidrug resistant (MDR) bacteria, such as methicillin-resistant Staphylococci aureus (MRSA), have evolved from hospital-acquired infections to community-acquired infections. Increasingly, MDR bacterial infections have the potential to cross the boundaries of hospital intensive-care units to those most susceptible.1-3 The global emergence and accelerated evolution of MDR bacteria has resulted in a call by researchers for more effective infection control measures in an attempt to halt their dissemination.2,4
It has long been recognized that the single most effective means of preventing the spread of disease is proper hand hygiene measures, which includes the use of protective gloves.5-7 Beginning in 1986, governmental organizations, such as the Centers for Disease Control and Prevention (CDC) and the Occupational Safety & Health Administration (OSHA), have recommended and mandated respectively the use of utility gloves as part of dental healthcare providers’ (DHCP) personal protective equipment (PPE) to prevent percutaneous and chemical injury during sterilization and disinfection procedures.8,9 Unlike disposable examination gloves, utility gloves are not considered a medical device and manufacturing standards are not regulated by the US Food and Drug Administration (FDA).5,8,9 Utility gloves are meant to protect DHCP from percutaneous/chemical injury rather than a means to prevent cross-contamination and/or cross-infection.5,8,9 There is no universally established protocol for donning, use, disinfection, and sterilization; protocols are largely designed and implemented by dental hospitals, academic dental clinics, and private dental practices with minimal guidance by those governmental and professional agencies that recommend and mandate their use.
A review of the literature detailed the evolution of handwashing and protective gloves as a means of infection control in healthcare. It also analyzed the elements of disease transmission, the role of resident and transient hand flora in cross-contamination/cross-infection, and the top five MDR bacteria as a possible underestimated reservoir for pathogenic bacteria. When utility gloves are used to carry out disinfection and sterilization procedures, they are donned with bare hands. The written policy, which follows governmental guidelines, instructs, “Utility gloves must be washed with antimicrobial soap, rinsed, and sprayed with a disinfectant after each use” should repeated use be anticipated in the same day.10 Used utility gloves are steam-autoclaved at the end of each day at 250 pounds per square inch for 20 minutes.
The “clean hand” technique implemented for donning and removing utility gloves requires multiple steps and can be repeated numerous times during a clinical day, increasing the risk of infection control error. As utility gloves are pulled on, the length of the cuffs extend beyond the length of examination glove cuffs to the contaminated sleeve of lab coats, increasing the risk of transferring bacteria to the inside of the utility gloves. The very act of washing utility gloves with soap and water may inadvertently allow for contamination. Water could travel the length of the glove, transporting bacteria from the outside to the inside via loose cuffs. The contaminated utility glove would then serve as a reservoir for bacteria, causing the recontamination of the DHCP’s hands with each subsequent use. The inside of utility gloves may provide an underestimated growth medium, given the literature’s verification that proliferation of bacteria increases rapidly in warm wet environments,11,12 combined with numerous other factors, such as the accumulation of hand sweat, inadvertent water contamination during the disinfection protocol, and the survival times of pathogenic bacteria on inanimate surfaces.13
It was theorized that this “perfect storm” of like conditions could diminish the safety for which their donning was intended to prevent. It is well established that dry or damaged hands can serve as a portal of entry as well as increase the risk of transient bacterial carriage and subsequent cross-contamination by way of a DHCP’s hands.5,14
No study was found to refute or support the presence or absence of pathogen bacteria on the inside of utility gloves. Four bacteria that accounts for 34% of all reported hospital-acquired infections were selected for the study.15 Because the environmental survival of pathogenic bacteria parallels the environmental survival of MDR bacteria of the same species, the presence of pathogenic bacteria found inside utility gloves served as an indication that environmental conditions equally favored the growth of MDR bacteria introduced into the same environment.12 A pilot study was conducted to lend empirical data and to help determine the need for the re-evaluation of the utility-glove protocol by answering the following questions:
1. After a day of use, at what frequency are gram-positive S aureus, K pneumoniae, E coli, and P aeruginosa present on the inside of used utility gloves?
2. To what degree are utility gloves contaminated?
3. Does the degree of contamination match the expected outcome?
METHODS AND MATERIALS
Institutional review board approval was granted. The researcher incurred all costs and no financial stakes from the design, conduction, or analysis of this pilot study were gained.
Each Wednesday for 6 weeks, five steam-autoclaved utility gloves from the clean utility-glove storage container were randomly selected to serve as control. A convenience sample of 10 used utility gloves placed in the sterilization area for sterilization following an 8-hour clinic day was selected for sampling. The randomness of the used utility-glove samples was defined by the random number of times the gloves were worn; the random size ranging from small, medium, large, and extra-large; the variation in hand-washing techniques; and the variation of unique bacteria found on individual hands.
Using aseptic technique, the inside of each utility glove was turned inside on a fabricated hand form to expose the index finger, palm area, and thumb. Using standard biological swabbing technique, a sterile swab moistened with sterile saline was used to collect a sample from each of the gloves. The sampling area originated from the index finger, continued from the index finger into palm area, and then extended to the tip of the thumb. The swab was used to inoculate the center area of two Fisher brand sterile 100-mm x 15-mm polystyrene Petri dishes containing Mannitol salt agar (MSA) and MacConkey agar. A new sterile swab moistened with sterile saline was used to uniformly distribute the inoculum on the MSA, employing a standard streak method. A second sterile swab moistened with sterile saline was used to distribute the inoculum on the MacConkey agar, employing the same streak method. Additionally, a Petri plate of MSA and MacConkey culture media were uncovered at the beginning of the sampling session and covered at the end of the session to serve as an airborne control.
The samples were incubated at 37ºC for 30 to 36 hours in aerobic conditions. Each plate was evaluated for CFUs. MSA is selective for salt-loving bacteria such as Staphylococci and differential in that pathogenic species of Staphylococci typically produce yellow colonies with yellow zones. Initially, S aureus was identified by colony morphology, gram stain, and the microscopic examination. Subsequent identification of S aureus was identified by distinct visual appearance of colony morphology on MSA. Gram-negative K pneumoniae, E coli, and P aeruginosa were identified by the distinct visual appearance on the selective and differential MacConkey culture media. CFU were counted up to 200 per Petri plate. The CFU counts were assigned a range of values to further qualify the degree of contamination expressed per Petri plate as shown in Table I.
ANALYSIS AND STATISTICS
Confidence intervals (CI) were constructed to estimate the rate of contamination. CIs were viewed as the probability that any randomly selected utility glove would express CFU contamination with a 95% confidence level (CL). Data collected from the pilot week of this pilot study were included in the statistical analysis because the results were consistent with the study data.
RESULTS
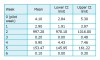
Rate of contamination: gram-negative K pneumoniae, E coli, and P aeruginosa: Petri plates of MacConkey agar expressed no growth for both steam-autoclaved utility gloves and used utility gloves. Table 2 summarizes the estimated rate of contamination expressed in CI for steam-autoclaved utility-glove controls and used utility-glove samples.
Degree of used utility-glove contamination: K pneumoniae, E coli, and P aeruginosa: No Petri plate of MacConkey agar expressed gram-negative CFU. Therefore, the degree of contamination could not be calculated.
Rate of contamination: gram-positive S aureus: Petri plates of MSA expressed growth for both steam-autoclaved utility gloves and used utility gloves. Table 3 summarizes the estimated rate of contamination expressed in CI for steam-autoclaved utility-glove controls and used utility-glove samples.
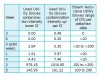
Degree of used utility-glove contamination: gram-positive S aureus: The degree of used utility-glove contamination was extremely varied over the 7-week sampling period. Therefore, the contamination rates were calculated separately for each of the sampling periods. The TNTC entries required an upper limit value to be included. A value of 1,400 CFU was assigned to TNTC. Table 4 presents the estimated mean intensity CFU with a 95% CL for each sampling period.
To further explore the relative intensity of used utility-glove samples, the chronology of weeks were arranged to identify perhaps three levels of contamination intensity as illustrated in Table 5. By comparing the lower upper CI limits with the mean, it is clear that there is a wide range of contamination from week to week. Arranged in this way, the intensity of contamination is at the lowest level in weeks 3 and 6, followed by weeks zero (pilot week), 1, and 4, with weeks 2 and 5 at the highest level of contamination intensity.
DISCUSSION
Frequency of used utility-glove contamination and expected outcomes: It was hypothesized that gram-negative culture media would not express growth of K pneumoniae, E coli, or P aeruginosa. No Petri plate expressed growth and, therefore, the raw date matched the expected outcome of zero. CIs based on 70 samples and a 95% CL estimated that the rate of contamination was no higher than 3%.
It was hypothesized that gram-positive culture media would express growth of S aureus but would not exceed the upper limits of the average carriage rate of 30% found in the general population in the United States.17 The raw data yielded a higher than expected outcome of 38.5%. CI, based on 70 samples, and a 95% CL, estimate the rate of contamination to be between 27% and 50%. However, the unexpected growth of S aureus from steam-autoclaved utility-glove controls confounded the used utility-glove sample results.
The raw data of steam-autoclaved utility gloves showed a contamination rate of 37.1%. CI, based on 35 samples and a 95% CL, estimate the rate of contamination to be between 23% and 53%.
Degree of contamination with S aureus: The raw data of steam-autoclaved utility-glove controls and statistical analysis of used utility-glove samples produced a wide variation of contamination levels ranging from under 20 CFUs to more than 200 CFUs per Petri plate. Beyond the degree of contamination, CIs suggest a wide variation in the intensity of contamination.
When the used utility-glove sample mean intensity CIs are paired with the corresponding week of raw steam-autoclaved utility-glove CFU control data, the contamination intensity and the range of contamination are closely matched (Table 6). The similarities of steam-autoclaved utility gloves to used utility-glove samples suggest the possibility of a correlation. It is reasonable to hypothesize steam-autoclaved utility-glove contamination was a contributing factor to the S aureus growth expressed from the used utility-glove samples. Additionally, the three levels of contamination shown in Table 5 suggest that there is some mechanism or process or event that occurs some weeks and not others that might explain the high level of variation between weeks.
Steam-autoclaved utility-glove contamination with S aureus: Weekly biological spore tests were conducted in the morning and utility-glove sampling was conducted in the afternoon of the same day. The spore test results indicated that all autoclaves were functional. It seems unlikely that functional steam autoclaves would kill highly resistant spores and not kill the less resistant Staphylococci bacteria. The possible mechanism, process, or event that preceded steam-autoclaved utility-glove contamination from functional autoclaves present concerns about the standard steam-autoclave sterilization procedures and the subsequent handling/storage of sterilized utility gloves. A number of possible contributing factors must be considered:
• Overloading the autoclave: Overloading may not allow for sufficient penetration for the utility gloves located closer to the middle of the autoclave.
• Length of time utility gloves were stored: Utility gloves were stored in a covered storage container over the summer. It is possible that the utility gloves became contaminated due to an extended period of storage.
• Condition in which utility gloves were stored: Utility gloves that were stored wet could have facilitated bacterial growth if S aureus was already present. It has also been shown that S aureus and MRSA have been recovered after periods of desiccation.12
• Airborne contamination: Airborne controls of MSA yielded a mean of 2.14 CFU per Petri plate for the 7-week trials.
• Damaged utility gloves: Damaged utility gloves, such as from tears, could provide an entry point for environmental S aureus contamination.
Alternatively, contamination could explain the expression of S aureus on culture mediate from samples taken from steam-autoclaved utility gloves. Given the technique-sensitive method of preparing, handling, and inoculation culture media, technique error cannot be ruled out.
STUDY LIMITATIONS
Steam-autoclaved utility gloves as “negative” controls: The study intended to evaluate the presence or absence of specific pathogenic bacteria inside utility gloves as a result of the protocol for donning and removing them during a day of clinical use. The contamination of steam-autoclaved utility-glove controls with S aureus confounded used utility-glove sample results.
The study design did not include controls to estimate the rate of sterile swab and sterile saline contamination. Culture media was prepared by the researcher and inspected for contamination prior to use. The number of contaminated culture media was recorded each week. The estimated rate of contamination of solid culture media preparation was evaluated with CI (Table 7).
Testing such as blood agar, alpha-hemolysis, coagulase activity, and catalase should have been conducted to further differentiate of S. aureus CFU on the MSA. There is no standardized method for sampling environmental surfaces largely due to the vast variety of surface areas chosen to sample by researchers. UMA Dental Health Programs provides four sizes of utility gloves: small, medium, large, and extra-large. The size variation helped to define the randomization of the utility gloves sampled but also served to weaken the strength of the study outcomes because the size of the surface area sampled inside the gloves varied corresponding to the size of the glove.
The sample size was small for CI to be constructed. The CIs would be narrower given a more precise estimate of the contamination rates. The arbitrary assignment of 1,400 CFU to any value beyond the CFU count of 200 for the purpose of measuring the intensity/degree to which utility gloves were contaminated did not accurately represent the true level of contamination and therefore, limits interpretation of the data represented in Tables 1, 5, and 6.
The emergence and dissemination of MDR bacteria begs a concerted effort by all healthcare providers to review and, if necessary, revise current infection control policies and procedures. The small sample size of this pilot study limits the conclusions that can be drawn. However, CIs indicate the risk of utility-glove contamination with gram-negative bacteria to be low. The findings of this study support current literature suggesting a low risk of transmission and/or infection with gram-negative bacteria in dentistry.16
Study design limitations and study design flaws notwithstanding, the unexpected contamination of steam-autoclaved utility gloves illuminate a potential gap in infection control. The ramifications of DHCP donning utility gloves contaminated with S aureus are unclear. However, steam-autoclaved utility gloves contaminated with S aureus may put DHCP at risk for infection and increase the risk of becoming hand carriers of pathogenic bacteria.7,17
Utility gloves, considered a nonmedical device, are not regulated by the FDA. Therefore, the quality of utility gloves varies by manufacturer specifications. This researcher found no studies in the literature evaluating the efficacy of utility gloves for their intended purpose of protecting DHCP from chemical and puncture injury nor were any studies found evaluating steam-autoclave effects and/or efficacy on utility glove material. The data collected from this pilot study can serve as an impetus for a more scientific and controlled study.
CONCLUSION
The risk of utility-glove contamination with gram-negative bacteria is low. The expressed growth of S aureus from steam-autoclaved utility-glove controls raises questions about the effectiveness and safety of generally accepted sterilization standards for the government-mandated use of utility gloves. Subsequent research should be conducted to more thoroughly differentiate, count, and statistically analyze microbial flora found on the inside of utility gloves. Research should also be conducted to determine if there are differences in material quality between manufacturers and to evaluate the effectiveness of steam-autoclave sterilization. In the era of evidence-based practice, the lack of studies representing the mandated use of utility gloves, combined with nonstandardized protocols, increases the potential risk of discrepancies in infection-control outcomes.
ABOUT THE AUTHORS
Kathy L. Grant RDH, BS, is a professional teacher for dental health programs at the University of Maine at Augusta, Bangor, and is a practicing dental hygienist. E. Donald Naber, EdD, is an associate professor of biological sciences and science coordinator at the University of Maine at Augusta, Bangor, and is an adjunct professor of nursing at the University of Maine. William A. Halteman, PhD, is a professor of biostatistics in the Department of Mathematics and Statistics at the University of Maine.
ACKNOWLEDGMENTS
The authors thank Dawn Bearor, EdD, for her insightful edits of the literature review that preceded this body of research.
REFERENCES
1. DePaola L, Fried J. Microbial resistance and health care-associated infections: combating this global threat part 1. Access. 2011;25(8):10-12.
2. DePaola L, Fried J. Microbial resistance and health care-associated infections: combating this global threat part 2. Access. 2011;25(10):22-24.
3. Ben-Ami R, Rodríguez-Baño J, Arslan H, et al. Multinational survey of risk factors for infection with extended-spectrum β-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682-690.
4. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159-166.
5. Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for Infection Control in Dental Health-Care Settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
6. Olsen RJ, Lynch P, Coyle MB, et al. Examination gloves as barriers to hand contamination in clinical practice. J Am Med Assoc. 1993;270(3):350-353.
7. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Infect Control Hosp Epidemiol. 2002;23(12 Suppl):S3-S40.
8. Model plans and programs for the OSHA bloodborne pathogens and hazard communications Standards [Internet]. Washington (DC): Occupational Safety and Health Administration; 2003 [cited 2014 April 23]; OSHA 3186-06R. Available from: https://www.osha.gov/Publications/osha3186.pdf.
9. Infection control in dental settings. FAQ. personal protective equipment [Internet]. Atlanta (GA): Centers for Disease Control and Prevention. Division of Oral Health. 2013 July 10 [cited 2014 April 23]. Available from: http://www.cdc.gov/OralHealth/infectioncontrol/faq/protective_equipment.htm.
10. University of Maine at Augusta-Bangor. Dental Health Programs. Clinic Manual: Section III: Infection control. 2013. 3 p.
11. Gould D, Chamberlain A. Gram-negative bacteria. The challenge of preventing cross-infection in hospital wards: a review of the literature. J Clin Nurs. 1994;3(6):339-345.
12. Cimolai N. MRSA and the environment: implications for comprehensive control measures. Eur J Clin Microbiol Infect Dis. 2008;27:481-493.
13. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130.
14. Gould D. Skin flora: implications for nursing. Nurs Stand. 2012;26(33):48-56.
15. Hidron A, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996-1011.
16. Laheij AMGA, Kistler JO, Belibasakis GN. Healthcare-associated viral and bacterial infections in dentistry. J Oral Microbiol. 2012;4.
17. Larson EL, Hughes CA, Pyrek JD. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26(5):513-521.