You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Inflammation is a normal process designed to protect and promote healing of injured tissues. It primarily consists of vascular events, but also includes cellular functions in concert with the immune system. Regardless of the nature of injury, the sequence of events is remarkably similar. A brief review of this process is essential to better distinguish the actions of steroidal versus nonsteroidal anti-inflammatory drugs (NSAIDs). By convention, the sequence of events is analyzed in terms of vascular and cellular phases, but realize that they occur simultaneously.
Vascular changes account for the familiar clinical signs of inflammation: redness, heat, pain, and swelling. Mechanical injuries to skin elicit a transient neural reflex resulting in vasoconstriction, but this response lasts only seconds and does not occur with many other types of injuries. Vasodilation and increased vessel wall permeability are the most consistent vascular responses. Vasodilation accommodates an increase in blood flow, ie, hyperemia, producing redness and heat. An increase in the permeability of vascular endothelium allows exudation of plasma, producing swelling and pain. Both of these vascular changes are brought about by local chemical mediators, ie, autacoids. These substances are either released by damaged cells or synthesized within the injured tissue, and include histamine, bradykinin, prostaglandins, and a variety of other complex agents. Some of these autacoids also sensitize sensory nerve endings and enhance nociception and pain transmission.
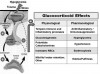
The cellular phase of inflammation commences when leukocytes adhere to the endothelial wall (margination), squeeze through the openings, and emigrate into the damaged tissues. Here the cells perform phagocytosis and other processes conventionally attributed to the immune response. These cells are summoned by a variety of chemical substances, a process called chemotaxis. Some of these chemotactic agents are the identical autacoids that mediate the vascular changes described above. Others are specific agents such as cytokines, synthesized solely for their chemotactic function, eg, eosinophilic chemotactic factor (Figure 1).
Although the inflammatory response is a normal protective process, its intensity and duration may become inappropriate and destructive, resulting in inflammatory disease. In this case, drugs having anti-inflammatory actions are indicated. Anti-inflammatory drugs interrupt the synthesis and/or release of mediators that initiate vascular changes and thereby suppress the cardinal signs described above. This action per se will not render an individual immunologically incompetent. Drugs that depress leukocyte function, especially lymphocytes, are designated more appropriately as immunosuppressant agents. In this regard, NSAIDs such as ibuprofen are anti-inflammatory, whereas glucocorticoids (those resembling cortisone) are both anti-inflammatory and immunosuppressant.
The NSAIDs have been reviewed in a previous continuing education article in this journal.1 In dental practice they are used mostly on a short-term basis, primarily for their analgesic effect. However, their impressive efficacy is also attributable to suppression of the inflammatory process that is a principal contributor. Both the analgesic and anti-inflammatory effects of NSAIDs are credited to their ability to inhibit synthesis of prostaglandins. They have less anti-inflammatory efficacy than the glucocorticoids, but their side effects are less severe. This is of particular importance if prolonged use is anticipated.
Physiological Functions of Glucocorticosteroids
The adrenal cortex is comprised of three cellular zones, each synthesizing a specific class of steroidal hormones. (The terms corticosteroid and corticoid are used interchangeably.) Their synthesis commences with cholesterol and culminates in the production of mineralocorticoids, glucocorticoids, and androgens. Aldosterone is the principal mineralocorticoid and functions in the conservation of sodium and water. Its synthesis and release are controlled by the angiotensin pathway and it has no additional metabolic or anti-inflammatory influences.2
Cortisol is the principal glucocorticosteroid and provides many physiological functions, including gluconeogenesis, which is the basis for its nomenclature. Like many endocrine organs, this zone of the adrenal cortex is under hypothalamic control and functions within the so-called hypothalamic-pituitary-adrenal axis. The hypothalamic-pituitary-adrenal axis and glucocorticoid effects are illustrated in Figure 2.
The synthesis and release of cortisol normally follows a diurnal rhythm in which the highest serum level appears in the morning hours and declines throughout the day until its inhibitory influence on corticotropin-releasing factor and corticotropin production is lost and a new cycle begins. Although 10 mg to 20 mg is the normal amount secreted daily, the cycle is altered when the hypothalamic-pituitary region is excited by stress, trauma, hypoglycemia, or other conditions that demand increased cortisol production.2,3
Anti-Inflammatory Action
In 1949, Hench et al4 discovered that high levels of cortisol in the blood of Cushingoid patients exerted an anti-inflammatory effect in a subset of patients also suffering rheumatoid arthritis. This was the first evidence that cortisol can produce anti-inflammatory effects. Until recently, this anti-inflammatory influence of glucocorticoids was believed evident only with therapeutic or supraphysiologic doses. More recent evidence has established that physiological levels of these hormones temper inflammation and immune functions, preventing them from becoming excessive and possibly destructive.2,3
Anti-inflammatory and most of the metabolic actions of glucocorticoids commence with their binding to specific receptors within the cytoplasm of targeted cells. The receptor-steroid complex then migrates into the nucleus, where it binds to DNA and alters genetic synthesis of proteins. Any number of cellular functions are thereby modified, including the production of enzymes that regulate myriad metabolic processes and those that regulate synthesis of inflammatory autacoids and immune-related cytokines.2,3,5 This mechanism is time consuming and accounts for a delayed onset of effect (6 to 8 hours) when glucocorticoids are administered clinically.
There also is accumulating evidence for so-called nongenomic actions of glucocorticoids in producing some of their additional effects, such as those in brain. Glucocorticoid excess leads to euphoria and psychosis, whereas deficiency results in lethargy, apathy, and depression. Some of these manifestations can occur within minutes of exposure and are thought to be mediated by as yet uncharacterized membrane-coupled receptors.2,3
To reiterate, the anti-inflammatory effects of glucocorticoids are largely due to a reduction in the synthesis and/or release of a variety of inflammatory mediators, including the prostaglandins that are also inhibited by NSAIDS. The sum of these actions results in suppression of vascular changes responsible for the cardinal signs of inflammation. Glucocorticoids also inhibit certain aspects of leukocyte function, which accounts largely for their immunosuppressant effect. They inhibit phagocytosis among macrophages and reduce the number and activity of specific subsets of T lymphocytes. They have less influence on humoral immunity, however. Existing antibody levels are not reduced significantly and B-cell response to antigen is not inhibited.2
Unfortunately, the supraphysiologic dosages required to produce an adequate anti-inflammatory effect will unavoidably result in a sobering list of untoward effects. When elevated serum concentrations are sustained, they not only suppress the hypothalamic-pituitary-adrenal axis, leading to adrenal atrophy, but also produce a series of exaggerated physiological responses summarized in Figure 2.
Considerations for Patients Receiving Chronic Therapy
The glucocorticoids have an enviable record in the management of primary inflammatory disorders, especially those attributed to immunologic mechanisms, eg, autoimmune disease, asthma, and rheumatoid arthritis. Their anti-inflammatory efficacy surpasses that of the nonsteroidal agents, eg, ibuprofen, but their potential for side effects is also greater. Although short-term use (1 week) is relatively safe, chronic use introduces many concerns regarding side effects.
Glucocorticoids inhibit osteoblast function, which is thought to account for osteoporosis that affects ~50% of patients treated for longer than 12 months.3 Osteonecrosis (also termed avascular necrosis) is also a well-recognized complication. This condition consists of a rapid and focal deterioration of bone quality and primarily affects the femoral head. Osteocyte apoptosis has been implicated in the pathogenesis of the condition, but there is still no explanation for individual susceptibility.2,3
For patients having severe disease, a physician may be forced to prescribe chronic therapy and accept the risk for side effects. We must assume that all patients receiving chronic supraphysiologic doses of glucocorticoids will have a compromised immune status and some degree of adrenal atrophy. When a surgical procedure is planned, there is increased risk for delayed healing or postoperative infection. In some cases, antibiotics may be ordered as a prophylactic measure. If complex extractions or placement of dental implants are planned, consideration must also be given to the possibility of steroid-induced osteoporosis and increased serum glucose concentrations associated with chronic glucocorticoid use. If present, these conditions may compromise treatment outcome.
While a patient is consuming a daily exogenous source of glucocorticoid, the patient’s adrenal cortex does not function, and this results in varying degrees of adrenal atrophy. This becomes a concern for patients receiving >15 mg/d of prednisone or its equivalent for >3 weeks.
The influence of smaller doses over longer durations is highly variable.2,3 When caring for these patients, the dental provider should assume there is at least some degree of impaired adrenal function. The prescribed steroid is not only therapeutic (anti-inflammatory), but it is also serving normal physiological requirements for the patient. This introduces two important considerations.
First of all, if the steroid medication is abruptly discontinued, the hypothalamus and pituitary will attempt to stimulate cortisol production in order to sustain normal cardiovascular function and glycemic control. However, the adrenal tissues will not respond, having atrophied during their sustained period of disuse. Common symptoms of acute adrenal insufficiency include irritability, nausea, arthralgia, dizziness, and hypotension. To avoid this complication, steroid medication must be withdrawn gradually, tapering the doses generally over 6 to 9 months to allow the atrophied cortex to regain functional status. In cases where oral intake is restricted and prevents normal medication consumption, an adequate amount of glucocorticoid should be administered intravenously during the preoperative period.
Secondly, patients who have not interrupted their medication may also present a concern. During particularly stressful periods, such as severe infection or surgery, additional steroid may be required to equal a cortisol surge that might have been produced by a functioning adrenal cortex. Arbitrary regimens for managing such patients generally consist of doubling or tripling the patient’s dose on the morning of surgery. Over the next 2 days, the dose is incrementally returned to baseline. For example, a patient taking 20 mg prednisone daily could be given 40 mg the day of surgery and 30 mg the day after surgery, and the normal 20-mg dosage could be resumed on the second day following surgery.
To lessen the adverse impact of chronic steroid therapy, the physician may attempt alternate-day steroid dosing. This schedule will permit the adrenal cortex to function on the drug-free day. Patients who are successfully managed in this manner will seldom develop significant adrenal atrophy or immunocompromise. However, daily doses of inhaled preparations should be considered to have risk similar to that with systemic administration.2,3
Use of Glucocorticoids in Dental Practice
Fortunately, the use of steroids in dental practice is only for brief periods, and carries little risk for those complications described with chronic use. ‘‘A single dose of glucocorticoid, even a large one, is virtually without harmful effects, and a short course of therapy (up to 1 week), in the absence of specific contraindications, is unlikely to be harmful.’’2 This view is confirmed by the use of astonishing doses of steroids administered to victims of acute spinal cord injury that improve neural recovery. In such cases, doses of methylprednisolone as high as 10 g are administered intravenously over a 24-hour period without significant side effects.6
The dental provider must appreciate that NSAIDs are effective anti-inflammatory agents for managing most cases of dental pain and should be regarded as first-line agents. However, there are several indications for which glucocorticoids either are preferable or may be considered when NSAIDs prove ineffective (Table 1).7-16 Mucosa lesions such as severe aphthous ulcerations or lichens planus are immune-mediated and require the added immunosuppressant actions of glucocorticoids. Both NSAIDs and glucocorticoids are effective for prophylaxis of postoperative nausea and vomiting, but the latter are more established.16-18 In other cases, steroids should be chosen when NSAIDs prove ineffective or when the event is anticipated to be severe, such as swelling following difficult third molar impactions.
The most common use of glucocorticoids in dental practice is to diminish the amount of postoperative swelling following surgical procedures.9-15 Clinical trials have confirmed the advantage of the preoperative administration of both NSAIDs and glucocorticoids over either agent alone.12-14 NSAIDs likely have a greater influence in reducing postoperative pain, whereas the glucocorticoids have a greater tendency to reduce postoperative swelling.12 Ideally, regimens should be initiated preoperatively and coverage extended postoperatively for the duration swelling is anticipated. This may be only a day or two for minor procedures, or as long as a week for more traumatic procedures. It should be clarified that this abbreviated use of glucocorticoids has not been found to increase the risk of postoperative infection, and the addition of antibiotic coverage solely for this purpose is unwarranted.
Glucocorticoids such as dexamethasone and methylprednisolone are well-established antiemetics for chemotherapy-induced as well as postoperative nausea and vomiting. How they produce this effect is unknown, but it is speculated that they suppress production of inflammatory autacoids that may somehow potentiate known vomiting pathways within the vomiting center. Management of postoperative nausea and vomiting has been reviewed in a previous continuing education article in this journal.18 Glucocorticoids have a slow onset of action, and their benefit is limited to prophylactic regimens.
Choice of Formulations
Glucocorticoids are strikingly similar in their molecular structures and clinical effects (Figure 3). Because of its low cost, prednisone is the most common medically prescribed steroid for chronic conditions. It is a pro-drug that must be biotransformed to the active metabolite, prednisolone. For this reason, it is only available for oral use that allows for first-pass metabolism during absorption. The various glucocorticoids are equivalent in anti-inflammatory efficacy and have similar side effect profiles, with the exception of fluid retention. Their major distinctions include potency (dose), duration, and mineralocorticoid (salt-retaining) activity. For these reasons, preparations are readily interchangeable provided equipotent doses are prescribed. Commonly used preparations are summarized in Table 2.
A given product is selected based primarily on the route by which it is to be administered and the duration of coverage desired. When treating mucosal lesions, topical ointments or rinses are generally preferred, but, when systemic administration is required, a 5-day regimen can be initiated using any one of several packaged formulations, such as the Medrol Dosepak. These formulations are arranged in rows of tablets corresponding to each day, with daily dosages reduced until the final day’s dosage is merely physiologic. Patients should be instructed to take each day’s tablets in the morning either at once or in two divided doses, once in the morning and once at noon. Evening doses should be discouraged because a surge in serum concentration at this time will mimic the height of normal circadian rhythm and lead to insomnia.3 Timing of doses should be altered accordingly for patients who work second or third shifts. A similar regimen is appropriate for managing traumatic neuritis of dental nerve trunks following surgery or local anesthetic injections, as well as phlebitis following intravenous sedation or anesthesia. In this latter case, a trial using NSAIDs and warm compresses should precede the use of glucocorticoids.
To counter postoperative swelling, one may elect to prescribe oral regimens described above or inject either conventional or long-acting repository formulations. Glucocorticoids prepared as standard phosphate salts can be injected by any route—intravenous, intramuscular, or submucosal—and provide a duration of effect approximating the biological half-lives presented in Table 2. The long-acting repository formulations are intended for intramuscular or intra-articular injection. These are absorbed slowly from the injection site and provide anti-inflammatory effects for 1 to 4 weeks, depending on the particular preparation. They are extremely irritating to tissues and must never be administered intravenously or near nerve branches exiting the mandible or maxilla. Suggested parenteral regimens are summarized in Table 3. Dexamethasone is a common choice for both postoperative nausea and vomiting and postoperative swelling and may produce a unique side effect unreported with other glucocorticoids. When dexamethasone is administered rapidly by intravenous infusion, patients may experience a bothersome genital or perineal burning or itch.19 No explanation has been offered for this effect, but it is less likely to occur if the medication is allowed to infuse slowly.
Summary
Compared to NSAIDs, glucocorticoids exhibit superior anti-inflammatory efficacy. This is particularly true for the medical management of immunologically mediated conditions, including graft rejection and autoimmune disease. Prednisone is the most widely used systemic preparation, but equipotent doses of other glucocorticoids are readily interchangeable. Chronic use is associated with a sobering list of adverse effects, but a few days, or even a week, of steroid therapy is generally free of significant side effects.2 Short-term use (5 to 7 days) of glucocorticoids in dental practice is unlikely to pose a risk for significant side effects. Although glucose levels and blood pressure may elevate slightly during treatment, short-term elevations are rarely of consequence. Nevertheless, several conditions should be regarded as relative contraindications for even short-term use. These include poorly controlled diabetes, immunocompromise, active peptic ulcer, osteoporosis, and active herpetic or fungal infections. It should also be mentioned that glucocorticoids produce poorly defined influences on mood and behavior. High dosages should probably be avoided in patients suffering psychoses or other severe affective disorders.20
About the Author
Dr. Becker is an Associate Director of Education, General Dental Practice Residency, at Miami Valley Hospital in Dayton, Ohio.
References
1. Becker DE. Pain management part 1: managing acute and postoperative dental pain. Anesth Prog. 2010;57:67-79.
2. Schimmer BP, Funder JW. ACTH, adrenal steroids and pharmacology of the adrenal cortex. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Companies Inc; 2011.
3. Stewart PM, Krone NP. The adrenal cortex. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa: Saunders Elsevier; 2011.
4. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24:181-197.
5. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723.
6. Bracken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. N Engl J Med. 1990;20:1405-1411.
7. Bahn SL. Glucocorticoids in dentistry. J Am Dent Assoc. 1982;105:476-481.
8. Silverman S Jr, Lozada-Nur F, Migliorati C, et al. Clinical efficacy of prednisone in the treatment of patients with oral inflammatory ulcerative diseases: a study of fifty-five patients. Oral Surg Oral Med Oral Pathol. 1985;59:360-363.
9. Beirne OR, Hollander B. The effect of methylprednisolone on pain, trismus, and swelling after removal of third molars. Oral Surg Oral Med Oral Pathol. 1986;61:134-138.
10. Montgomery MT, Hogg JP, Roberts DL, Redding SW. The use of glucocorticosteroids to lessen the inflammatory sequelae following third molar surgery. J Oral Maxillofac Surg. 1990;48:179-187.
11. Mico-Llorens JM, Satorres-Nieto M, Gargallo-Albiol J, et al. Efficacy of methylprednisolone in controlling complications after impacted lower third molar surgical extraction. Eur J Clin Pharmacol. 2006;62:693-698.
12. Sisk AL, Bonnington GJ. Evaluation of methylprednisolone and flurbiprofen for inhibition of the postoperative inflammatory response. Oral Surg Oral Med Oral Pathol. 1985;60:137-145.
13. Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of nonsteroidal anti-inflammatory drugs, ibuprofen and flurbiprofen, with methylprednisolone and placebo for acute pain, swelling, and trismus. J Oral Maxillofac Surg. 1990;48:945-952.
14. Buyukkurt MC, Gungormus M, Kaya O. The effect of a single dose prednisolone with and without diclofenac on pain, trismus, and swelling after removal of mandibular third molars. J Oral Maxillofac Surg. 2006;64:1761-1766.
15. Moore PA, Brar P, Smiga ER, Costello BJ. Preemptive rofecoxib and dexamethasone for prevention of pain and trismus following third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:E1-E7.
16. Rawlinson A, Kitchingham N, Hart C, et al. Mechanisms of reducing postoperative pain, nausea and vomiting: a systematic review of current techniques. Evid Based Med. 2012;17:75-80.
17. De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114:424-433.
18. Becker DE. Nausea, vomiting and hiccups: a review of mechanisms and treatment. Anesth Prog. 2010;57:150-157.
19. Neff SP, Stapelberg F, Warmington A. Excruciating perineal pain after intravenous dexamethasone. Anaesth Intensive Care. 2002;30:370-371.
20. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169:491-497.