You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Two of the major public health problems of the aged population today are osteoporosis and periodontal disease. The social and financial costs of bone fractures and tooth loss are common among the aged.1 The cost to the U.S. health system is estimated to be approximately $20 billion annually for emergency calls, surgical treatment, physiotherapy and rehabilitation, time missed from work, and emotional distress due to impaired lifestyles.2
Osteoporosis is a systemic disease involving loss of bone mineral density, resulting from an imbalance between bone formation and resorption.3 Osteoporosis and related fractures are the primary health care concern in America, being more prevalent than coronary disease, stroke and breast cancer.4 Postmenopausal osteoporosis is the most common form of osteoporosis. The risk of fracture increases exponentially after menopause, manifesting itself in wrist fractures after the age of 50, vertebral fractures after the age of 60 and hip fractures after the age of 70. The Report of the United States Surgeon General states that half of all American citizens older than 50 will be prime candidates for low bone mineral density by 2020, predicting that 1 in 3 women will be affected.5
Unlike osteoporosis, periodontal disease is a localized inflammatory response to bacteria in the mouth, causing alveolar bone loss. Because the number of older people in the population worldwide has increased and more of these older adults are retaining their teeth, the potential for greater prevalence of periodontal disease is increasing.2
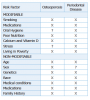
Interest in the relationship between osteoporosis and periodontal disease has increased over the years. The influence of osteoporosis on the progression of periodontal disease was not studied until Groen’s report in 1968.6 Recently, investigations linking oral and systemic diseases have become popular in the medical and dental fields. Both osteoporosis and periodontal disease occur more frequently after the age of 35, and they share several risk factors (Table I).7
Knowing the role that osteoporosis plays in the destruction of alveolar bone may help to identify methods which would be useful for diagnosing both osteoporosis and periodontal disease.8 The American Academy of Periodontology considers that postmenopausal osteoporosis is a risk factor for periodontal disease.9 On the other hand, osteoporosis is not an etiologic factor in periodontal disease, but may affect the severity of pre-existing periodontal disease.10 The purpose of this comprehensive literature review is to summarize the scientific evidence, examining the relationship between postmenopausal osteoporosis and periodontal disease, and to determine if the relationship is causal or casual.
Methods and Materials
The search strategy consisted of identifying key terms: osteoporosis, periodontal disease, alveolar bone loss, estrogen deficiency, tooth loss and postmenopausal. Literature was searched in 8 databases: PubMed, CINAHL, Web of Science, Google Scholar, Cochrane Library, Melvyl, PsychINFO and NCBI. Over 520 articles were identified and screened for potential inclusion on the basis of their abstract. Inclusion criteria limited studies to dentate postmenopausal women and periodontal disease. Additional articles were identified from reference lists of selected articles. The full texts of 107 articles were read for thorough examination. The types of studies included in this review were longitudinal, cross-sectional and case-control studies.
Review of the Literature
This literature review identified and examined the scientific evidence, which had investigated the relationship between osteoporosis and periodontal disease. The authors found that researchers had used a variety of methods in those studies, both in their study designs and their assessments of osteoporosis and periodontal disease. Because methods affect results and conclusions, it is critical to understand the methodology of the studies. Therefore, this review will begin with an explanation and evaluation of the methods to assess osteoporosis and periodontal disease.
Assessment of Osteoporosis
The disease condition of osteoporosis is usually determined by a measurement of bone mineral density (BMD). BMD is expressed in terms of the number of standard deviations (SD) from the mean of healthy individuals, matched to age and sex (the Z-score), and the number of SD from the mean of healthy young sex-matched individuals (the T-score).11 According to the World Health Organization, osteoporosis is considered to be present when BMD is 2.5 SD below the BMD of the young normal individual. Osteopenia is defined as bone density levels between 1 SD and 2.5 SD below normal BMD.11 Fracture risk is approximately doubled for every 1 SD below the young adult mean BMD.12
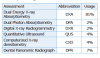
There are several tools available to measure BMD (Table II). The most widely recognized is dual-energy x-ray absorptiometry (DXA). Non-invasive DXA is reliably used around the world to identify patients with low BMD because of its high precision and resolution, high accuracy, low radiation dose, and low cost.13 Although it requires visiting a separate facility, which can be inconvenient, it remains the gold-standard assessment of osteopo-rosis. Dual-photon absorptiometry (DPA) is similar in concept to DXA, however, it is not as advantageous because it has a longer scan time and shorter source life.7 Prior to the development of computerized densitometry, digital x-ray radiogrammetry (DXR) was used. This less precise technique estimates BMD by evaluating a standard radiograph of the hand.14 A less common assessment test used by 2 studies in this review is the Quantitative Ultrasound (QUS) of the calcaneal and phalanges.15,16 It provides a measure of skeletal status by determining a Stiffness Index (SI), a measure of bone strength, which is sensitive to bone structure.
Another type of osteoporosis assessment method measures the thickness of the mandibular inferior cortex (MIC) below the mental foramen on a dental panoramic radiograph (DPR).17 The cortical bone was chosen over the trabecular bone due to its greater consistency among readings, which may be due to trabecular bone being more easily influenced by dental infections.18 The porosity of the MIC is classified using the Klemetti Index (Table III).19 This classification system is an excellent means to determine undiagnosed osteoporosis.20 Based on MIC findings of erosion or thin cortical width on DPR, younger postmenopausal women could be identified as osteoporotic.21 A decrease in MIC thickness by 1 mm was shown to increase the likelihood of osteoporosis by 47%.22 Mild to moderate MIC erosion on the DPR correctly reflected the presence of osteoporosis 83% of the time, and a normal MIC reading predicted a normal BMD 60% of the time.18 This means that normal spine BMD would correlate with normal MIC evaluations on DPR greater than half the time. This method has great potential because DPRs are taken as part of routine dental examinations.
Assessment of Periodontal Disease
Many dental conditions affect the postmenopausal age group, including tooth loss and periodontal disease and prevalence increases with age.2 In the reviewed studies, periodontal disease was assessed with a diversity of outcome measures (Table IV). In general, studies lacked concise and widely accepted assessment criteria for diagnosing periodontal disease, making comparisons among studies and conclusions challenging. Gomes-Filho proposed a gold standard of the combination of periodontal bone resorption (>3 mm) with 3 other clinical descriptors for the disease: pocket depth (PD) (>4 mm), clinical attachment level (CAL) (>3 mm) and bleeding upon probing (BOP).23 These 3 clinical descriptors had the greatest frequency among the reviewed studies, with probing depth used 17% of the time, CAL 13% and BOP 15%, confirming that Gomes-Filho made a logical choice. Using a standardized grouping of these 3 clinical outcome measures in future studies would facilitate studying the association between periodontal disease and osteoporosis.
Investigations Studying the Relationship
Common risk factors between osteoporosis and periodontal disease can be both modifiable and non-modifiable (Table I). Examining the relationship between the 2 diseases requires addressing these factors as well as the type of study design. Studies will be discussed according to the type of study design used by investigators: longitudinal, cross-sectional and case-control.
Investigations using the Longitudinal Study Design
Five longitudinal studies were reviewed (Table V), all of which used DXA as an osteoporosis assessment, and 4 showed an association between osteoporosis and periodontal disease. In a recent study, the recurrence of periodontitis among women who had been non-surgically treated for periodontal disease occurred with greater severity in women with osteoporosis than in those without osteoporosis.24 Studies by Jonasson found that mandibular trabecular patterns could be used as an indicator of osteoporosis.25 They demonstrated that skeletal bone loss was associated with a decrease in alveolar bone mass and that less alveolar bone was a highly significant predictor of future fracture risk. The long observation time of 10 years in this study makes the results highly credible. The study conclusions also suggest that DPRs could be used to screen patients prior to recommending further osteoporosis testing.26 In the Hildebolt study, after 3 years of hormone replacement therapy, osteoporotic postmenopausal women had significant increases in their alveolar crestal height (ACH).27 This increased bone mass was observed throughout the body, indicating the positive relationship between systemic and oral bone loss.
One study did not show a relationship between osteoporosis and periodontal disease. Famili studied a total of 253 dentate women, and found no difference in absolute or percentage change in BMD between women with or without periodontal disease.28 The lack of association did not seem to result from a lack of reproducibility of probing depth and recession/hyperplasia measurements because the intra-examiner kappa index indicated significant reliability. Another explanation for the findings may be that the population was older and had greater numbers of missing teeth, possibly due to periodontal disease that occurred earlier in life.
Investigations Using the Cross-sectional Study Design
The 25 cross-sectional studies are listed in sequential order beginning with the most recent (Table VI). The majority of these studies (81%) used DXA as the measurement of BMD, while the periodontal disease assessments varied greatly. Twenty cross-sectional studies showed an association between osteoporosis and periodontal disease with varying degrees of significance. The studies of most interest were those using DPR to determine MIC classification of oral bone loss. Using this technique, the likelihood of osteoporosis was increased by a 1 mm decrease in MIC thickness.22 Another study found that mild to moderate MIC erosion was associated with osteoporosis 83% of the time.18
Another approach with potential to link osteoporosis and periodontal disease was the study of cytokines, the presence of which was observed in both diseases.8 Cytokines, such as receptor activator of nuclear factor K B ligand (RANKL) and osteoprotegerin (OPG), play a critical role in the production of bone-resorbing osteoclasts. Their presence in both conditions illustrates the common mechanism of osteoclast formation and bone resorption. Other biochemical markers of bone turnover, such as serum C-terminal telopeptides, have also been identified in both conditions. Levels were higher in an osteoporotic postmenopausal population, as compared to a non-osteoporotic population.22 The NHANES III study found that calculus played a significant role in a 3-way interaction with CAL and BMD.29 Women with the highest calculus scores exhibited an inverse association between BMD and CAL. Thus, these data demonstrate a relationship between more severe periodontal disease and greater systemic bone loss.
Modifiable lifestyle factors influenced the association of BMD and tooth loss (Table IV). More damaging behaviors, such as smoking, were related to less number of teeth and less bone.30 Dentition count was related specifically to cortical bone in skeletal sites.15 Loss of posterior teeth related positively to low BMD of the spinal column.2 The same results were found in the large osteodent study including 665 women in 4 European centers, and confirmed an association between osteoporosis and having less than a full complement of teeth.31 Inagaki used the criterion of at least 20 teeth, because that number had been set as a major goal of the national oral health campaign in Japan, called 80-20 (80 year-olds retaining 20 teeth).32 Even with a maximum of teeth, osteoporosis was present. That study also confirms that low BMD may not become evident until past the age of 80, and that the reason for tooth loss may not be known. This makes it difficult to use tooth loss alone as an accurate measurement in the analysis of periodontal disease.
A total of 10 studies that showed an association between osteoporosis and periodontal disease were similar in their statistical analysis, although not always similar in their use of assessments. The most recent study, conducted in 2011, confirmed a significant association of age and years since menopause with BMD, showing the importance of specifying those criteria in osteoporosis studies. These postmenopausal women exhibited severe periodontal disease, which was significantly associated with osteoporosis.33 Alveolar bone density of the maxilla and mandible showed highly significant positive correlation with DXA T-scores, demonstrating the same effects of both disease processes.16 Three other studies with similar multivariate analyses found >3 times the likelihood of ACH loss in postmenopausal women with osteoporosis.34-36 The association was stronger in women 70 to 85 years of age, compared to subjects <70 years of age, which confirms the age-related findings in the previously discussed studies by Sultan33 and Vishwanath.16 Four cross-sectional studies had the periodontal assessment of CAL in common. Postmenopausal women with osteoporosis had a 2.5 times greater risk of having periodontal disease than women without osteoporosis, confirming the previous findings regarding ACH.37 Osteoporotic women presented with higher CAL values than those with normal BMD, while CAL measurements of osteopenic women did not differ from those with normal bone density levels.38 This would suggest that early diagnosis of low BMD prior to confirmed periodontal disease may be beneficial to prevent periodontal disease. Another study also found that osteoporotic sites had significantly higher interproximal CAL values than non-osteoporotic sites in postmenopausal women.7,39 The statistical results of all these studies suggest osteoporosis as a risk indicator for periodontal disease in postmenopausal women.
Of the 25 cross-sectional studies, 5 did not show an association between osteoporosis and periodontal disease. In 3 of these 5 studies, the number of teeth was not related to systemic BMD.40-42 This may indicate that the number of teeth may not be relevant in the assessment of a relationship. Peri-implantitis was not found to be associated with osteoporosis, but peri-implantitis is a very specific class of periodontal disease with specific bacteria.43 In another study, the MIC assessments significantly differed between normal and osteoporotic groups, however, the authors attributed this difference to functional demands of occlusion on bone remodeling in the mandible.44 If the local effects of occlusion and the attached muscle, as well as smoking, had been factored out, the results might have demonstrated a more convincing difference.
Investigations Using Case-control Study Design
The 3 case-control studies matched postmenopausal women with osteoporosis to women with normal bone levels (Table VII). Postmenopausal women with a history of osteoporotic fractures tended to have increased resorption and thinning of the mandibular inferior cortex, as compared to their matched controls.17 In postmenopausal women with low educational levels, subjects with osteoporosis were predisposed to more severe periodontal disease than their matched controls without osteoporosis.1,23 Case-control studies have the potential to assess associations between an exposure (osteoporosis) and an outcome (periodontal disease), but not whether the exposure preceded or caused the outcome. This is a limitation of the case control study design.
Conclusion
This comprehensive literature review demonstrates the possible association between postmenopausal osteoporosis and periodontal disease: postmenopausal women with low systemic BMD tended to have greater loss of alveolar bone and clinical attachment. Four reasonable hypotheses for these results include destructive lifestyle risk factors, susceptible genetic factors, increased production of inflammatory mediators and less initial BMD in both systemic and oral bones.7However, the evidence is inadequate to determine the most likely hypothesis.
Demonstration of a relationship between osteoporosis and periodontal disease is complex because both are multifactorial diseases sharing multiple risk factors.43 A major issue affecting the ability to relate osteoporosis with periodontal disease is the lack of uniformity in diagnosing periodontal disease. Specific criteria have not been established, as evidenced by the fact that 21 different assessments in a variety of combinations had been used in the studies of this review. Because these different outcome measures influence the definition of periodontal disease, determining whether periodontal disease is associated with osteoporosis may not currently be feasible. A precise measurement of periodontal disease is needed to validate investigations on this topic.1Furthermore, well-designed longitudinal studies, addressing all these factors, are recommended to determine whether the relationship between osteoporosis and periodontal disease is causal.
Based on the findings of this study, cross-communication and patient referral between medical and dental professionals are recommended to improve the health of the postmenopausal public. The radiographic information gathered in dental offices can be used to screen patients for undiagnosed low BMD of the jaws, not to diagnose osteoporosis. The goal is to recognize the potential risks for low BMD and potential fracture, and to refer patients to their medical doctor.45 Collaborative actions for preven-tion, evaluation and treatment of oral diseases and osteoporosis in postmenopausal patients can offer benefits in terms of reduced tooth loss, less periodontal disease and less loss of BMD. These outcomes would yield a healthier life for postmenopausal women, ultimately reducing co-morbidities and oral health care costs.
References
1. Passos Jde S, Gomes-Filho IS, Vianna MI, et al. Outcome measurements in studies on the association between osteoporosis and periodontal disease. J Periodontol. 2010;81(12):1773-1780.
2. Henriques PS, Pinto Neto AM. Association between tooth loss and bone mineral density in brazilian postmenopausal women. J Clin Med Res. 2011;3(3):118-123.
3. Kaye EK. Bone health and oral health. J Am Dent Assoc. 2007;138(5):616-619.
4. Edwards BJ, Migliorati CA. Osteoporosis and its implications for dental patients. J Am Dent Assoc. 2008;139(5):545-552.
5. U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Office of the Surgeon General. U.S. Department of Health and Human Services. 2004.
6. Groen JJ, Menczel J, Shapiro S. Chronic destructive periodontal disease in patients with presenile osteoporosis. J Periodontol. 1968;39(1):19-23.
7. Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71(9):1492-1498.
8. Jabbar S, Drury J, Fordham J, Datta HK, Francis RM, Tuck SP. Plasma vitamin D and cytokines in periodontal disease and postmenopausal osteoporosis. J Periodontal Res. 2011;46(1):97-104.
9. Scannapieco FA. Position paper of the American Academy of Periodontology: Periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69(7):841-850.
10. Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(10):1041-1049.
11. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1-129.
12. Wasnich R. Bone mass measurement: Prediction of risk. Am J Med. 1993;95(5A):6S-10S.
13. Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000. 2000;23:94-102.
14. Swezey RL, Draper D, Swezey AM. Bone densitometry: Comparison of dual energy x-ray absorptiometry to radiographic absorptiometry. J Rheumatol. 1996;23(10):1734-1738.
15. Drozdzowska B, Pluskiewicz W, Michno M. Tooth count in elderly women in relation to their skeletal status. Maturitas. 2006;55(2):126-131.
16. Vishwanath SB, Kumar V, Kumar S, Shashikumar P, Shashikumar Y, Patel PV. Correlation of periodontal status and bone mineral density in postmenopausal women: A digital radiographic and quantitative ultrasound study. Indian J Dent Res. 2011;22(2):270-276.
17. Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Case-control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(4):518-524.
18. Taguchi A, Sanada M, Krall E, et al. Relationship between dental panoramic radiographic findings and biochemical markers of bone turnover. J Bone Miner Res. 2003;18(9):1689-1694.
19. Klemetti E, Kolmakov S, Kroger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994;102(1):68-72.
20. Persson RE, Hollender LG, Powell LV, et al. Assessment of periodontal conditions and systemic disease in older subjects. I. focus on osteoporosis. J Clin Periodontol. 2002;29(9):796-802.
21. Taguchi A, Tsuda M, Ohtsuka M, et al. Use of dental panoramic radiographs in identifying younger postmenopausal women with osteoporosis. Osteoporos Int. 2006;17(3):387-394.
22. Vlasiadis KZ, Damilakis J, Velegrakis GA, et al. Relationship between BMD, dental panoramic radiographic findings and biochemical markers of bone turnover in diagnosis of osteoporosis. Maturitas. 2008;59(3):226-233.
23. Gomes-Filho IS, Passos Jde S, Cruz SS, et al. The association between postmenopausal osteoporosis and periodontal disease. J Periodontol. 2007;78(9):1731-1740.
24. Gomes-Filho IS, Oliveira TJ, Passos JS, et al. Effect of osteoporosis on periodontal therapy among post-menopausal women. Gerodontology. 2013;30(1):40-48.
25. Jonasson G. Bone mass and trabecular pattern in the mandible as an indicator of skeletal osteopenia: A 10-year follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(2):284-291.
26. Jonasson G, Jonasson L, Kiliaridis S. Changes in the radiographic characteristics of the mandibular alveolar process in dentate women with varying bone mineral density: A 5-year prospective study. Bone. 2006;38(5):714-721.
27. Hildebolt CF, Pilgram TK, Yokoyama-Crothers N, et al. The pattern of alveolar crest height change in healthy postmenopausal women after 3 years of hormone/estrogen replacement therapy. J Periodontol. 2002;73(11):1279-1284.
28. Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol. 2005;76(1):11-15.
29. Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: Cross-sectional evaluation of US adults from NHANES III. J Clin Periodontol. 2000;27(10):778-786.
30. Gur A, Nas K, Kayhan O, et al. The relation between tooth loss and bone mass in postmenopausal osteoporotic women in turkey: A multicenter study. J Bone Miner Metab. 2003;21(1):43-47.
31. Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, et al. Tooth loss and osteoporosis: The osteodent study. J Clin Periodontol. 2009;36(3):190-197.
32. Inagaki K, Kurosu Y, Yoshinari N, Noguchi T, Krall EA, Garcia RI. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in japanese women. Calcif Tissue Int. 2005;77(1):9-14.
33. Sultan N, Rao J. Association between periodontal disease and bone mineral density in postmenopausal women: A cross sectional study. Med Oral Patol Oral Cir Bucal. 2011;16(3):e440-e447.
34. Al Habashneh R, Alchalabi H, Khader YS, Hazza’a AM, Odat Z, Johnson GK. Association between periodontal disease and osteoporosis in postmenopausal women in jordan. J Periodontol. 2010;81(11):1613-1621.
35. Brennan-Calanan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Osteoporosis and oral infection: Independent risk factors for oral bone loss. J Dent Res. 2008;87(4):323-327.
36. Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76(11):2116-2124.
37. Lopes FF, Loureiro FH, Pereira Ade F, Pereira AL, Alves CM. Association between osteoporosis and periodontal disease. Rev Bras Ginecol Obstet. 2008;30(8):379-383.
38. Pepelassi E, Nicopoulou-Karayianni K, Archontopoulou AD, et al. The relationship between osteoporosis and periodontitis in women aged 45-70 years. Oral Dis. 2012;18(4):353-359.
39. Shen EC, Gau CH, Hsieh YD, Chang CY, Fu E. Periodontal status in post-menopausal osteoporosis: A preliminary clinical study in taiwanese women. J Chin Med Assoc. 2004;67(8):389-393.
40. Kulikowska-Bielaczyc E, Golebiewska M, Preferansow E. The relationship between mineral status of the organism and the number of teeth present and periodontal condition in postmenopausal patients. Adv Med Sci. 2006;51:130-133.
41. Lundstrom A, Jendle J, Stenstrom B, Toss G, Ravald N. Periodontal conditions in 70-year-old women with osteoporosis. Swed Dent J. 2001;25(3):89-96.
42. Slaidina A, Soboleva U, Daukste I, Zvaigzne A, Lejnieks A. Postmenopausal osteoporosis and tooth loss. Stomatologija. 2011;13(3):92-95.
43. Dvorak G, Arnhart C, Heuberer S, Huber CD, Watzek G, Gruber R. Peri-implantitis and late implant failures in postmenopausal women: A cross-sectional study. J Clin Periodontol. 2011;38(10):950-955.
44. Naitoh M, Kurosu Y, Inagaki K, Katsumata A, Noguchi T, Ariji E. Assessment of mandibular buccal and lingual cortical bones in postmenopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(4):545-550.
45. Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):349-356.
About the Authors
Diane Z. Dodd, RDH, MS
Private Practice
Mount Diablo, California
Dorothy J. Rowe, RDH, MS, PhD
Associate Professor Emeritus, Department of Preventive and Restorative Dental Sciences University of California, San Francisco