You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Current imaging techniques in the dental office are essentially 2-dimensional representations of 3-dimensional (3D) objects.1-4 However, with new information and technology appearing almost weekly, cone-beam computed tomography (CBCT) has gained considerable acclaim worldwide in recent years as a viable 3D imaging modality. To date, most applications of CBCT have been in the fields of implantology, oral surgery, dental radiology, and orthodontics.5 Improved spatial resolutions and more user-friendly software programs have opened the door for more clinicians than ever before to use this technology. Nonetheless, the question remains: Are dentists ready to use CBCT in endodontics?
Although a survey by dental radiologists showed that most CBCT scans were performed for implant treatment planning (49%),5 popularity of endodontic applications does not seem far away. Researchers have shown that CBCT has tremendously greater sensitivity in detecting apical periodontitis, when compared to periapical and panoramic radiographs.6 In a population of 888 consecutive patients (1,508 teeth) with endodontic infection, the prevalence of apical periodontitis, when comparing panoramic and periapical radiographs and CBCT, was 17.6%, 35.3%, and 63.3%, respectively (P < .001).6 With such a strong impact already suggested in the literature, this article describes CBCT and its practicality as a diagnostic tool in clinical endodontic practice.
How Is a CBCT Scan Performed?
There are some universal concepts to consider when operating CBCT units. The patient is seated or stood in a CBCT unit, similar to a panoramic radiography unit, between an x-ray source and an image detector. Relevant patient information is entered into the software program, and the area of the head to be scanned is positioned. Upon starting the scan, there is a cone-shaped beam of x-ray radiation that is emitted from a source to the patient. The “shadow” of that patient is then cast onto a detector. The source and detector together move in one 360° rotation around the patient, capturing a 3D “shadow” of the patient. This 3D volume of captured data is, essentially, a cylinder of 3D pixels called voxels, which vary in dimensions depending on the manufacturer and scan settings selected by the operator. Basically, the smaller and more numerous the voxels, the better the spatial resolution.
At this point, there is still no image to be seen on the computer screen. The data have to be reconstructed, using one of many available CBCT software programs. Each software program uses specific algorithms (eg, Feldkamp algorithm7) to assemble the 3D volume data on a computer and further manipulate that image. Now a CBCT image appears.
This is where the power of CBCT can be harnessed. Not only can the image be rotated in any direction but also its intrinsic properties can be explored. In other words, the software can virtually peel away the bone to show the dentition or vice versa. This property of all matter was originally outlined by Sir Godfrey Hounsfield.8 His work, which is still shown, assigned a unit value to matter: air is -1000, water is 0, and hard tissue with ascending higher values (Table 1). Values higher than 1000, usually metallic objects, create a scatter phenomenon when scanned, which will be reviewed later in the article.9,10
 |
How Accurate Is a CBCT Image?
Articles discussing micro-computed tomography sparked immediate interest among dental researchers, especially in the area of rotary file design. Bench-top micro-computed tomography (CT) allowed groundbreaking research, which has shown, despite the use of endodontic rotary file systems, 35% to 50% of the root canal system remained completely untouched.11 The researchers conducted their study by scanning extracted teeth before and after instrumentation and calculating the relative percentage of unchanged voxels from the original canal shape. This procedure, however, was done using 34-µm resolution, so only voxels ≥ 0.34 µm can accurately be identified. Any changes < 34 µm would not be registered as changed voxels.
At approximately 125-µm spatial resolutions, current CBCT units are four times “blurrier” than their micro-CT counterparts. Hence, the previous study cannot be replicated clinically with the same level of accuracy using CBCT. Furthermore, the level of radiation is exponentially greater for a micro-CT scan than a CBCT scan, despite providing only about four times better resolution.
The question can then be asked: Can linear measurements still be made in a 3D object accurately using CBCT, despite poorer spatial resolution? Several researchers, using standardized objects12 or human specimens,13-16 have shown CBCT to be very accurate to 1 to 2 voxels.
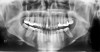
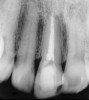
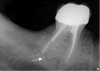
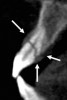
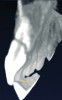
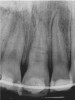
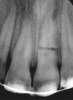
In addition to linear accuracy, it has been suggested that CBCT can have diagnostic accuracy with regards to periapical lesions.17-19 In one of these studies,17 periapical lesions were analyzed with CBCT, then surgically excised and biopsied to compare with histology. The authors found comparable results. Although the study showed promising results with CBCT, the researchers conceded that histology remains the gold standard in the diagnostic armamentarium. In another study,19 the ability to identify periapical lesions was assessed using radiographs and spiral CT. With CT, 100% of lesions were identified, while only 78% were identified with radiographs alone. Concomitantly, the location of the lesions proximal to the inferior alveolar nerve was visualized more readily with CT (100% vs 39%). Figure 1A through Figure 1E illustrate a case in which an endodontically treated tooth appeared normal on panoramic and periapical radiographs. Only CBCT showed the presence of apical pathology.6
Is There Clinical Evidence to Support CBCT Use in Endodontics?
Complicated anatomy of both teeth and bone, periapical pathology, periodontal-endodontic communications, as well as other treatment planning and diagnostic considerations, make any additional information, 3D imaging in particular, essential to present the best possible treatment options to patients.
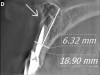
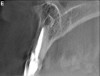
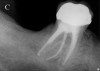
Initially, case studies appeared in the endodontic literature in which patients underwent CBCT imaging for the purposes of diagnosis20-22 and presurgical treatment planning.19 Recent reports have successfully shown the use of CBCT to locate missed canals,23 detect the extent of dentoalveolar fractures,20 identify resorption patterns,22 and compare cystic with granulomatous periapical lesions.17 Figure 2A through Figure 2C show a case in which the missed canal was suggested by periapical radiographs but confirmed with CBCT.24 Similarly in Figure 3A through Figure 3D, the additional diagnostic information attained from CBCT confirmed the diagnosis and extent of the root fracture, which initially was observed in the periapical radiographs (Figure 4A and Figure 4B).20 Notably, the extent of the lingual fracture as well as any alveolar complications could be visualized by CBCT only.
The limitation is the spatial resolution that is used. At best, today’s CBCT equipment can capture images at 125 µm; however, there is growing demand for an improved resolution, closer to the resolution obtained with bench-top micro-CT units. The problem with increasing the resolution is that the radiation dose absorbed by the patient also increases. This will have to be addressed by both CBCT manufacturers and dentists.
The absorbed radiation dose delivered to patients remains paramount in the treatment rendered in dentistry. The US Nuclear Regulatory Commission set the radiation protection guidelines, which state that the amount of radiation exposure should be as low as reasonably achievable (ALARA). This is based on the “linear hypothesis” theory of radiation.25 According to this concept, the amount of radiation absorbed is additive and cumulative throughout the life of a patient or healthcare worker. With respect to CBCT, the amount of radiation for a full head-and-neck scan may be roughly equivalent to a full-mouth set of radiographs, depending on the manufacturer and scan setting26-29 (Figure 5). This dosage drops considerably with limited-view scanning modes, which are now available from many CBCT-unit manufacturers. The limited-view modes allow practitioners to scan specific quadrants of the mouth, which not only reduces the radiation dose but also accelerates image capture and reconstruction. As previously mentioned, an inverse-relationship balance exists between radiation dose and resolution.
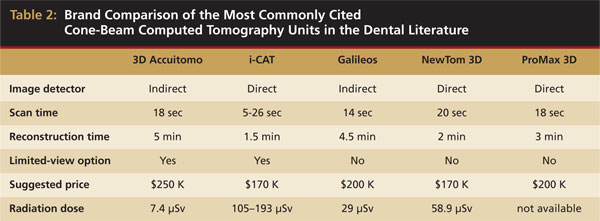
When reviewing the literature, each study notes the specific manufacturer and CBCT unit used. Table 2 shows a comparison of the five most commonly cited manufacturers in the literature, along with some notable features of each unit. The image detectors available are both charge-coupled detectors (CCD), which is an indirect method, and the newer direct image detectors. The table also notes the wide range of radiation doses delivered to patients.
 |
Are There Any Other Disadvantages of CBCT?
There are several obstacles for clinicians who are interested in CBCT technology but have not yet been able to implement it into their practices. Aside from the high initial purchase price, there are other critical issues that must be considered before use. As with any major equipment purchase, the benefits must outweigh the costs.
First, the purchase of a CBCT unit, which can cost more than $150,000, must be justified. With each scan costing the patient $300 to $600, enough scans must be performed to recover the cost of the equipment. In addition, indirect costs, such as data storage, staff training, time, and space, also must be considered. Scheduling changes also may be required. With several patients seen each day, an office with only one or two staff members may experience CBCT scanning as a constant interruption to treatment time.
For use in endodontic purposes, the resolution and radiation-level exposure for the patient still need improvement. Moreover, the scatter radiation that is well documented in the literature remains a problem in attaining a clean image using CBCT. As previously mentioned, this phenomenon occurs when objects greater than 1000 Hounsfield units are scanned, primarily metallic restorations, orthodontic appliances, etc. Also, vertical root fractures cannot be identified readily by CBCT.26 At the currently available resolutions, not only could the fracture line be too small but interference from surrounding structures also may hinder visualization.
There are also medical and legal concerns that accompany all forms of imaging, not just CBCT. The dentist is responsible for any and all information that can be rendered on each radiograph taken or CBCT scan performed in the office. For example, for a complete CBCT image of the head and neck region, the clinician must be ready to differentiate normal anatomy from abnormal anatomy. In particular, the clinician must be able to recognize calcified carotid atheromas at the level of C3 and C4 vertebrae when present.30 Therefore, a dentist must develop a relationship with a dental radiologist for both improved patient care and to minimize missed diagnoses.
Furthermore, CBCT may show detail that dentists do not wish to see. Are dentists ready to expose their work to such 3D scrutiny? With the ability to visualize the periapical status of teeth postoperatively with such clarity, could this possibly reduce the reported success rates in endodontic therapy?
What’s on the Horizon?
As previously mentioned, improved technology should produce an image with increased resolution and a concomitant reduction in radiation emitted. In addition to improvements in resolution, perhaps to levels closer to bench-top micro-CT (approximately 34 µm), there also will be advances in algorithms and further automation of procedures. This could include automatic tracing of the inferior alveolar nerve in 3D and location of other regional anatomy such as sinuses and calculation of linear distances. Furthermore, the amount of data storage required must be reduced to facilitate e-mailing of images. Currently, one full-view CBCT image may encompass as much as 50 megabytes of space, which can be transferred to a CD.
There also must be a reduction in overall technology cost. With several brands on the market and a multitude of software platforms, competition will most likely meet clinician-cost demands in the long run.
These are long-term goals for an exciting technology that is at its infancy in clinical dentistry. Only practitioner feedback and demand for this technology will drive industry improvements. In addition, clinician determination and a desire to provide the most advanced care to patients will undoubtedly aid in implementation of CBCT into dental practice.
In conclusion, the authors feel that the many obstacles to CBCT are far surpassed by the technology’s potential to improve the care rendered to patients. In endodontics, further improvements in this technology should be followed closely in the coming years.
References
1.Bender IB, Seltzer S. Roentgenographic and direct observation of experimental lesions in bone: I. J Am Dent Assoc. 1961;62:152-160.
2.Bender IB, Seltzer S. Roentgenographic and direct observation of experimental lesions in bone: II. J Am Dent Assoc. 1961;62:708-716.
3.van der Stelt PF. Experimentally produced bone lesions. Oral Surg Oral Med Oral Pathol. 1985;59(3):306-312.
4.White SC, Atchison KA, Hewlett ER, et al. Efficacy of FDA guidelines for prescribing radiographs to detect dental and intraosseous conditions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(1):108-114.
5.Suomalainen AK, Salo A, Robinson S, et al. The 3DX multi image micro-CT device in clinical dental practice. Dentomaxillofac Radiol. 2007;36(2):80-85.
6.Estrela C, Bueno MR, Leles CR, et al. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008;34(3):273-279.
7.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1(6):612-19.
8.Hounsfield GN. Computed medical imaging. Science. 1980;210(4465):22-28.
9.Katsumata A, Hirukawa A, Noujeim M, et al. Image artifact in dental cone-beam CT. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(5):652-657.
10.Miles DA, Danforth RA. A clinician’s guide to understanding cone-beam volumetric imaging (CBVI). 2007. The Academy of Dental Therapeutics and Stomatology Web site. http://www.ineedce.com/pdf_files/ACliniciansGuide.pdf. Accessed May 20, 2008.
11.Peters OA, Laib A, Göhring TN, et al. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod. 2001;27(1):1-6.
12.Marmulla R, Wortche R, Muhling J, et al. Geometric accuracy of the NewTom 9000 Cone Beam CT. Dentomaxillofac Radiol. 2005; 34(1):28-31.
13.Hilgers ML, Scarfe WC, Scheetz JP, et al. Accuracy of linear temporomandibular joint measurements with cone beam computed tomography and digital cephalometric radiography. Am J Orthod Dentofacial Orthop. 2005;128(6):803-811.
14.Misch KA, Yi ES, Sarment DP. Accuracy of cone beam computed tomography for periodontal defect measurements. J Periodontol. 2006;77(7):1261-1266.
15.Pinsky HM, Dyda S, Pinsky RW, et al. Accuracy of three-dimensional measurements using cone-beam CT. Dentomaxillofac Radiol. 2006;35(6):410-416.
16.Lascala CA, Panella J, Marques MM. Analysis of the accuracy of linear measurements obtained by cone beam tomography (CBCT-NewTom). Dentomaxillofacial Radiology. 2004;33(5):291-294.
17.Simon JHS, Enciso R, Malfaz JM, et al. Differential diagnosis of large periapical lesions using cone-beam computed tomography measurements and biopsy. J Endod. 2006;32(9):833-837.
18.Lofthag-Hansen S, Huumonen S, Gröndahl K, et al. Limited cone-beam CT and intraoral radiography for the diagnosis of periapical pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(1):114-119.
19.Velvart P, Hecker H, Tillinger G. Detection of the apical lesion and the mandibular canal in conventional radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(6):682-688.
20.Cohenca N, Simon JH, Roges R, et al. Clinical indications for digital imaging in dento-alveolar trauma. Part 1: traumatic injuries. Dent Traumatol. 2007;23(2):95-104.
21.Cohenca N, Simon JH, Mathur A, et al. Clinical indications for digital imaging in dento-alveolar trauma. Part 2: root resorption. Dent Traumatol. 2007;23(2):105-113.
22.Cotton TP, Geisler TM, Holden DT, et al. Endodontic applications of cone-beam volumetric tomography. J Endod. 2007;33(9):1121-1133.
23.Huumonen S, Kvist T, Gröndahl K, et al. Diagnostic value of computed tomography in re-treatment of root fillings in maxillary molars. Int Endod J. 2006;39(10):827-833.
24.Cotton TP, Geisler TM, Holden DT, et al. Endodontic applications of cone-beam volumetric tomography. J Endod. 2007;33(9):1121-1132.
25.Cohen BL. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995; 68(2):157-174.
26.Patel S, Dawood A, Ford TP, et al. The potential applications of cone beam computed tomography in the management of endodontic problems. Int Endod J. 2007;40(10):818-830.
27.Hashimoto K, Kawashima S, Araki M, et al. Comparison of image performance between cone-beam computed tomography for dental use and four-row multidetector helical CT. J Oral Sci. 2006;48(1):27-34.
28.Ngan DC, Kharbanda OP, Geenty JP, et al. Comparison of radiation levels from computed tomography and conventional dental radiographs. Aust Orthod J. 2003;19(2):67-75.
29.Scarfe WC, Farman AG, Sukovic P. Clinical applications of cone-beam computed tomography in dental practice. J Can Dent Assoc. 2006;72(1):75-80.
30.Friedlander AH. Calcified carotid artery atheromas. J Am Dent Assoc. 2007;138(9):1191-1192.
About the Authors
Royeen Nesari, DDS, Clinical Endodontic Instructor, University of California, San Francisco, San Francisco, California
Louis E. Rossman, DMD, Clinical Professor, University of Pennsylvania Department of Endodontics, Philadelphia, Pennsylvania
Samuel I. Kratchman, DMD, Associate Clinical Professor, University of Pennsylvania Department of Endodontics, Philadelphia, Pennsylvania