You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
All-ceramic crowns have become the predominant choice for fixed dental prostheses. In a 2019 study conducted by The National Dental Practice-Based Research Network, of the 3,468 single-unit crowns evaluated, 70% were fabricated from a ceramic material.1 Therefore, it is more important than ever for the clinician to be familiar with contemporary dental ceramic materials..
What Is a Ceramic?
Ceramics are one of the major classifications of dental materials. In fact, all indirect restorative materials can be classified as either polymers, metals, ceramics, or some combination of these. In polymers, the atoms are arranged in discrete chains of molecules. Metals and ceramics, on the other hand, have their atoms arranged in lattices, which are repeating geometric arrangements of atoms. The lattices of atoms in metals are held together by metallic bonds, whereas the lattices of atoms in ceramics are held together by ionic bonds. Although the metallic bonds that hold metals together allow the planes of atoms to slide past each other when a force is applied, the ionic bonds that hold ceramics together do not, and forces applied to ceramics may cause the bonds between atoms to separate.2 So, why is this important in dentistry? When a patient places a heavy occlusal force on a gold crown, the result is a slight deformation of the crown (ie, planes of atoms sliding past each other). Alternatively, when a heavy occlusal force is placed on a porcelain veneer, the result is a crack in the veneer (ie, ionic bonds separating). A ceramic material's tendency to crack rather than deform is referred to as its brittleness. Brittleness is not the same as strength, which is calculated from the force required for a material to fracture. Despite the fact that ceramics will not deform prior to fracturing, some are very strong. When restoring with ceramics, a major consideration is choosing a ceramic material that is strong enough for the specific location in the mouth to not crack under function.
Ceramics are formed from the combination of a metallic element and a nonmetallic element, which is commonly oxygen. To name ceramics, the name of the metallic element from which they are derived is changed by substituting its suffix with the letter "A." For example, the ceramic formed from the metallic element zirconium is referred to as zirconia (ie, zirconium dioxide). A common point of confusion is the misidentification of zirconia as a metal material. It is not; it is a ceramic material due to its lack of metallic bonds and as evidenced by its brittle nature.
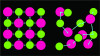
The atoms in a ceramic can be arranged in a lattice that has a perfectly ordered structure, which is described as a crystal. However, there can be atoms within the lattice that disrupt the perfect crystalline arrangement, which are referred to as flux. When the crystalline structure of the lattice is disrupted, the arrangement of atoms becomes disorganized or "amorphous" (Figure 1). Ceramics with an amorphous arrangement of atoms are referred to as glasses.2 These two types of arrangements of atoms in ceramic materials, crystalline and glassy, affect two of their most important characteristics related to dentistry: strength and translucency.
When there is a perfect crystalline arrangement, more atoms may be packed into a space. Therefore, crystalline ceramics possess a higher density of atoms with a higher density of ionic bonds between them when compared with glassy ceramics. When a crack is started in a crystalline ceramic restoration, such as a zirconia crown, it is more difficult for it to propagate because there are many bonds that need to be broken. Conversely, cracks more easily propagate through glassy ceramic restorations, such as porcelain veneers, because there is a lower density of bonds to break.2
The tradeoff with glassy ceramic materials is that their lower density of atoms allows more light to pass through without interference. When more light passes through a ceramic, it makes the ceramic more translucent. For this reason, glassy ceramics, such as porcelain, generally exhibit a more enamel-like translucency than crystalline ceramics.2 However, there are some exceptions to this tendency, including crystalline ceramics that possess an interatomic spacing that allows more light to pass through, such as cubic zirconia.3
In addition to differences in strength and translucency, glass-based ceramics differ from polycrystalline ceramics in the way in which they may be bonded. To roughen glass-based ceramics prior to bonding, they may be etched with hydrofluoric acid. Acid etching removes some of the glass and exposes crystals. These exposed crystals provide surface roughness to interlock with resin cement. Polycrystalline ceramics, such as zirconia, cannot be etched due to their lack of glass; therefore, surface roughness is achieved through sandblasting.
Dental ceramics may be classified as those that contain predominantly glass, those that are a mix of glassy and crystalline components, and those that are all crystalline.4
Mostly Glass Ceramics
The terms porcelain and ceramic are sometimes erroneously used interchangeably, but porcelain is a type of ceramic that contains mostly glass. This error may stem from use of the CDT code D2740 Crown - porcelain/ceramic substrate. Feldspathic porcelain is a naturally occurring version of porcelain comprising kaolin, quartz, and feldspar. Dental porcelain is predominantly composed of an amorphous glassy phase; however, there are also some leucite crystals added to adjust its coefficient of thermal expansion and increase its strength.
Although porcelain veneers have an impressive clinical record when bonded to enamel,5currently, porcelain is primarily used to cover more opaque framework materials, such as metal and zirconia copings. Porcelain may be applied by hand in a powder/liquid form. Alternatively, a wax layer can be fused to a coping, invested in stone, and burned out, after which porcelain is pressed into the investment.
Porcelains that are designed to layer over zirconia copings have been formulated to better match the coefficient of thermal expansion of zirconia.6 In addition, when porcelain is fired onto a zirconia coping, it must be removed from the furnace slowly because zirconia is a thermal insulator that loses heat slower than a metal coping.7 If the layered crown is removed too quickly, cracks can form in the porcelain because there is a differential cooling rate between the porcelain in contact with the zirconia coping and the porcelain exposed to the external environment. Using the proper porcelain and a slow cooling process can help reduce the incidence of chipping among porcelain-layered zirconia crowns.
Glass-Ceramics
Glass-ceramics are ceramics that are composed of a significant amount of crystalline phase reinforcing a glassy matrix. Historically, leucite-based ceramics were one of the original glass-ceramic materials. Although leucite ceramic crowns may still be used by some clinicians who prefer their esthetic properties, the combined strength and translucency of lithium disilicate has propelled this newer material to predominance.1 Lithium disilicate is a generic name for glass-ceramics that contain lithium oxide as the predominant oxide other than silica. Lithium disilicate materials start as a precursor glass in which crystals are nucleated (ie, originated) and grown through the application of heat. Some manufacturers immediately crystallize lithium disilicate to its full potential whereas others only partially crystallize the blanks and allow full crystallization to occur during the heating step of the restoration fabrication process. Placing lithium disilicate restorations in a furnace following milling will also heal any cracks that may have formed during the milling process.8
Recently, there have been several different formulations of lithium disilicate materials produced by different manufacturers. There is a distinction that can be made between their chemical compositions because some are true lithium disilicates, which contain Li2Si2O5; some are lithium silicates, which contain Li2SiO3; and others are combinations that contain both chemistries. More important than the chemical composition, however, is the microstructure of the resulting crystals. The function of crystals within a glass-ceramic is to divert the paths of cracks to prevent them from growing, which increases the strength of the material. Crystals with a higher aspect ratio (ie, more elongated) are better able to divert cracks and contribute to higher strength materials (Figure 2).9
Lithium disilicate restorations may be fabricated by milling (ie, ground/cut from a blank with a diamond bur) or pressed (ie, waxed, invested, burned out, and heat injected into the investment). Because these processes are so different, the crystalline microstructure varies between the milled and pressed versions of glass ceramics of the same brand. In fact, the milled versions of some glass-ceramic materials have significantly lower strength properties than the pressed versions of the same materials.9
A recent trend in lithium disilicate materials is the addition of trace amounts of zirconia to improve translucency; however, the addition of zirconia has been theorized to contribute to a thickening of the glassy phase of lithium disilicates, which can limit the growth of lithium disilicate crystals. Furthermore, zirconia has been credited for increasing crystal nucleation site density, which also limits crystal growth. Decreased crystal growth can reduce the strength of glass-ceramic materials.10
Polycrystalline Ceramics
In the past, alumina was widely used as a high-strength polycrystalline ceramic coping material. The improved properties of zirconia, however, have rendered alumina crowns mostly obsolete. Although zirconia was initially used only as a coping material, ongoing improvements to its optical properties allowed it to be used as a monolithic crown material, which ultimately lead to its mass popularization. The first modification to the initial formulation of zirconia to improve its optical properties was to reduce the amount of added alumina from 0.25% to 0.05%.11 This improvement in translucency is what began zirconia's transition from a coping material to a monolithic crown material.
Several manufacturers began to designate the reduced-alumina content zirconia as high translucency zirconia; however, the material was still much more opaque than glass-ceramics. To further improve the translucency of zirconia, manufacturers began adding cubic phase zirconia. Cubic phase refers to an atomic arrangement of the zirconia crystals that is more symmetrical and allows more light through than the original atomic arrangement, which is referred to as the tetragonal phase. Cubic phase zirconia is created by increasing the yttria content. Adding 5 mol% of yttria (5Y) creates approximately 50% cubic zirconia and adding 4 mol% yttria (4Y) creates approximately 25% cubic zirconia, whereas adding 3 mol% yttria (3Y) results in zirconia that is mostly void of the cubic phase.10 In communications between clinicians, laboratories, and manufacturers, zirconia is designated based on its yttria content in order to describe its relative translucency. For example, 5Y zirconia has the highest content of cubic phase and is the most translucent version of zirconia.
Unfortunately, the drawback of increasing the cubic phase and reducing the tetragonal phase of zirconia is that it leads to a lower strength material. The tetragonal crystals of zirconia impart strength through a process known as transformation toughening. In transformation toughening, cracks formed in tetragonal zirconia are healed when the crystals around them expand, compress them, and halt their progression (Figure 3). Although 3Y zirconia possesses this crack-healing property and 4Y zirconia is capable of some crack healing, 5Y zirconia demonstrates a very limited ability to heal cracks through transformation toughening.12
Zirconia restorations also differ from glass-ceramic ones in the techniques used to fabricate them. Dental zirconia is produced by compressing a fine powder into a puck- or block-shaped blank that exhibits a chalk-like consistency. The blank is milled into an oversize restoration, which is then sintered in a furnace. During the sintering process, heat fuses the zirconia powder into a dense structure, and the restoration shrinks to its correct dimensions. Because zirconia is milled in a chalk-like consistency and into an oversize shape, it is easier to mill than glass ceramics. However, the sintering temperature of zirconia is much higher than the crystallization temperature of glass-ceramics; therefore, zirconia requires a specific furnace.
Conclusion
Dental ceramics may be classified on a spectrum from those that contain mostly glass phase to those that contain all crystalline phase. Ceramics that contain more glass phase are more translucent, but those that are more crystalline phase are stronger. Porcelain is a mostly glass ceramic that is predominantly layered over more opaque ceramics to improve translucency, whereas glass-ceramics (eg, lithium disilicates) contain a much higher percentage of the crystalline phase, which helps to prevent crack propagation. Although zirconia is a polycrystalline material, it is fabricated with different proportions of tetragonal and cubic phase crystals that lead to varying levels of translucency and strength. A thorough understanding of the properties of available dental ceramics allows clinicians to select the most appropriate materials for restorative situations based on the specific balance of esthetics and functional strength they require.
About the Authors
Nathaniel Lawson, DMD, PhD
Director
Associate Professor
Department of Biomaterials
School of Dentistry
University of Alabama at Birmingham
Birmingham, Alabama
Preshtha Mangla, BDS, MS
Resident
Department of Biomaterials
School of Dentistry
University of Alabama at Birmingham
Birmingham, Alabama
References
1. Lawson NC, Litaker MS, Ferracane JL, et al; National Dental Practice-Based Research Network Collaborative Group. Choice of cement for single-unit crowns: findings from The National Dental Practice-Based Research Network. J Am Dent Assoc. 2019;150(6):522-530.
2. Powers JM, Wahata JC. Dental Materials: Foundations and Applications. 11th ed. Elsevier; 2017.
3. Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014;30(10):1195-1203.
4. Giordano R II. Ceramics overview. Br Dent J. 2022;232(9):658-663.
5. Watt E, Conway DI. Review suggests high survival rates for veneers at five and ten years. Evid Based Dent. 2013;14(1):15-16.
6. Rodrigues CS, Dhital S, Kim J, et al. Residual stresses explaining clinical fractures of bilayer zirconia and lithium disilicate crowns: a VFEM study. Dent Mater. 2021;37(11):1655-1666.
7. Tan JP, Sederstrom D, Polansky JR, et al. The use of slow heating and slow cooling regimens to strengthen porcelain fused to zirconia. J Prosthet Dent. 2012;107(3):163-169.
8. Phark JH, Duarte S Jr. Microstructural considerations for novel lithium disilicate glass ceramics: a review. J Esthet Restor Dent. 2022;34(1):92-103.
9. Lubauer J, Belli R, Peterlik H, et al. Grasping the lithium hype: insights into modern dental lithium silicate glass-ceramics. Dent Mater. 2022;38(2):318-332.
10. Hallmann L, Ulmer P, Kern M. Effect of microstructure on the mechanical properties of lithium disilicate glass-ceramics. J Mech Behav Biomed Mater. 2018;82:355-370.
11. Zhang Y, Lawn BR. Novel zirconia materials in dentistry. J Dent Res. 2018;97(2):140-147.
12. Zhang F, Reveron H, Spies BC, et al. Trade-off between fracture resistance and translucency of zirconia and lithium-disilicate glass ceramics for monolithic restorations. Acta Biomater. 2019;91:24-34.