You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The maxillary lateral incisor is the second most common congenitally missing tooth.1 Agenesis of this tooth is estimated to occur in 1.5% to 1.8% of the population.2 Additionally, it is one of the most commonly lost teeth due to trauma. As a result, the replacement of the missing maxillary lateral incisor remains a persistent challenge for dental clinicians. In 2005,Kokich and Kinzer discussed the three classic treatment options for replacement of the missing lateral incisor: canine substitution, implant, and pontic.3-5 More recently, two additional treatment possibilities have been described: autotransplantation and a long-term interim restoration on a temporary anchorage device (TAD).6 All of these treatment options have intrinsic risks and benefits.
Treatment Options
Canine Substitution
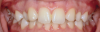
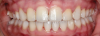
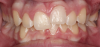
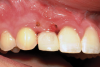
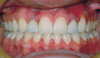
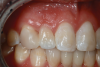
Canine substitution can be an excellent alternative for the replacement of the missing maxillary lateral incisor (Figure 1 and Figure 2). This treatment option can be particularly effective if the canine has a flat facial surface, is not too wide mesiodistally, and has a color similar to the contralateral lateral incisor. Patients with missing lateral incisors who present with maxillary dentoalveolar protrusion and/or an Angle class II molar relationship and minimal crowding in the mandibular arch are considered excellent candidates for canine substitution.7
Implants
Replacement of the missing lateral incisor with an implant has become a primary treatment option in the past two decades. Advantages of the implant include longevity and the ability to maintain arch length. However, there are significant financial costs and risks associated with this treatment option. First, in the cases of agenesis and trauma, the soft tissue and underlying bone often may be inadequate for implant placement, thus requiring soft- and/or hard-tissue grafting. A second risk is the long-term stability of the bone and soft tissue overlying the facial surface of the implant. If an implant is placed in a young adult, it is reasonable to assume that the patient may live for another 50 to 60 years or more. If the soft tissue and bone thin out over that lengthy amount of time, both functional and esthetic failures may arise. One study found blueish discoloring of the gingiva in 50% of single-implant crowns at 4 years post-treatment due to thinning of the facial alveolar bone and overlying gingiva.8
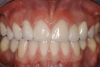
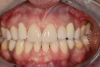
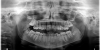
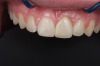
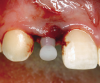
A third risk of using an implant to replace a missing lateral incisor is the continued vertical growth of the alveolar bone and eruption of the teeth adjacent to the implant.9,10 Traditionally, it has been taught that an implant can be safely placed when alveolar bone growth is confirmed complete with serial radiographs. However, numerous studies call that rule into question.11-16 Bernard et al evaluated vertical changes in teeth adjacent to implants in a young group of patients (15.5 to 21 years) and in a mature group (40 to 55 years), over a mean time of 4.2 years.17 In the young group, infraocclusion of the implant crowns ranged from 0.1 mm to 1.65 mm, while in the mature group the infraocclusion of the implant crowns ranged from 0.12 mm to 1.86 mm. An example of infraocclusion that occurred in a young implant patient over time is depicted in Figure 3 and Figure 4. Based on classic literature as cited here, it seems prudent to delay the placement of implants in high-risk areas, such as the maxillary lateral incisor region, for as long as possible or perhaps avoid it altogether.
Autotransplantation
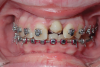
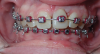
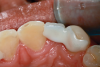
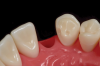
A less commonly used but highly effective method of replacing lost anterior teeth is autotransplantation.18 A common indication for autotransplantation is the replacement of a missing maxillary anterior tooth with a mandibular second premolar. (A case example is shown in Figure 5 through Figure 10.) Timing of the surgery is critical. Orthodontic treatment must be timed so that the recipient site is ready when the donor tooth has two-thirds to three-fourths root development. Measurements must be made to ensure that the crown width of the donor tooth is comparable to the contralateral incisor of the tooth being replaced.
A socket is surgically created in the recipient site, and the donor tooth is atraumatically extracted; the entire dental follicle must be intact and undamaged. The donor tooth is placed in the newly created socket. There must be no pressure on the periodontal ligament, and the transplanted tooth must be out of occlusion. The tooth is stabilized for 6 weeks with a light orthodontic wire. As the tooth matures, it may erupt normally; however, orthodontic treatment is frequently required to move the tooth into the correct position for restoration.
The risks associated with autotransplantation are ankylosis, pulpal necrosis, and inflammatory or replacement resorption. However, Andreasen et al reported that when performed properly with attention paid to detail, the success rate of autotransplantation is above 90%.18 A recent systematic review also found tooth transplantation with a follow-up of 6 years or more to be successful.19 The authors of the review reported survival rates ranging from 75.3% to 91%. The percentage of ankylosis ranged from 4.2% to 18.2%, and the percentage of root resorption ranged from 3% to 10%. Reasons for failure include maturation of the donor tooth, surgical technique, initialization stabilization of the transplanted tooth, and orthodontic movement of the transplanted tooth.
Temporary Anchorage Device
A novel approach for the replacement of the maxillary lateral incisor is the use of a TAD to support a composite or porcelain crown. This technique can provide an esthetic result for the short term or long term and is relatively inexpensive. However, the TAD does integrate into the alveolar bone and theoretically has the potential to impede the long-term vertical growth of the alveolus. Cope and McFadden published a case report of two patients who received TAD restorations and were followed-up at 99 months and 27 months.6 No impediment of vertical growth was reported in either case. (A case example is depicted in Figure 11 through Figure 14.)
An alternative technique intended to avoid the potential complication of impeding the long-term vertical growth of the alveolus was reported on by Ciarlantini and Melson, in which the TAD was placed in the palate, perpendicular to the alveolar process, approximately corresponding to the coronal third of the length of the roots of the adjacent teeth.20 A wire was then attached to the TAD, which extended over the edentulous site. A composite crown was then constructed on the end of the wire directly in the mouth. In a 5-year follow-up of six restorations, the authors reported continued vertical development of the alveolar process in the edentulous sites.
Pontic
Yet another option for the replacement of a missing maxillary lateral incisor is the pontic. In 1973, Rochette first described a bonded metal splint for mandibular anterior teeth.21 In 1977, Howe and Denehy discussed the bonded metal framework to replace missing teeth.22 By 1982, when Livadtitis and Thompson described the Maryland bridge,23 the use of a pontic had become a well-accepted technique. When bonded to enamel, metal substrate bonded bridges could be very successful.24
However, there were two primary problems. First, when bonded to the lingual surfaces of the abutment teeth, the metal substrate would lower the value of the abutment teeth, resulting in unesthetic gray teeth coloring. Second, one of the two bonded wings commonly would de-bond. The bridge would remain in place with one bonded wing, but the abutment with the unbonded wing would often develop caries. When this de-bonding occurred, the strategy was to remove the unbonded wing and leave the bonded bridge in place with only one wing. Briggs et al and Kern and Glaser were early proponents of the single-wing technique.25,26 In time, it became apparent that bonded bridges with only one wing were more successful than those with two wings. In 2017, Kern et al reported a 98.2% survival rate on 108 zirconia bonded bridges at 10 years.27 Sailer and Hämmerle had similar success with a mean follow-up time of 53 months.28 Research has also shown that bonded bridges were more successful when resistance form (ie, vertical grooves or boxes) was incorporated into the technique.29
To overcome the graying of the abutment teeth, techniques using composite and low-strength porcelain frameworks were introduced. However, neither of these options provided the required strength for predictable longevity. The problem of fracture resistance was resolved with the advent of zirconium dioxide, or zirconia, used as the substrate. When used in bonded bridges, zirconia provides the esthetics of porcelain and the strength of a metal substrate.27 Initially, the challenge with zirconia was the development of a predicable bonding protocol; however, with current zirconia materials this problem has been overcome.
Over the past two decades, zirconia has become a primary material used in fixed prosthodontics. The first generation of zirconia, 3Y-TZP (3 mol % yttria-stabilized tetragonal zirconia polycrystalline), is the strongest with a flexural strength of 1300 MPa to 1500 MPa.30 However, it is also the most opaque zirconia. Because of its strength and high opacity, it serves well as a framework for more esthetic veneering materials and for monolithic restorations in areas of the mouth where high-level esthetics are not required. A subsequent generation of zirconia, 5Y-TZP, is significantly more translucent but much weaker; therefore, it should not be used as a framework material for bonded bridges.31
Debate among dental professionals regarding the validity of bonding to a zirconia substrate persists.32 However, according to a 2015 systematic review, the technology to successfully bond to zirconia exists.33 Zirconium dioxide (Y-TZP) is a glass-free ceramic that is formed by directly sintering crystals together. It cannot be conventionally etched with hydrofluoric acid; therefore, other adhesion strategies must be utilized.34
Bonding to zirconia requires two essential steps. First the intaglio surface must be cleaned prior to bonding. This can be accomplished using a product specifically designed to remove proteins from this surface. Alternatively, the intaglio surface may be abraded with airborne particle abrasion using 50-µm aluminum oxide. This cleans the contaminated intaglio surface and increases surface roughness, surface energy, and wettability.35 The second essential step to bonding to zirconia is using a resin cement that incorporates the MDP monomer (10-methacryloyloxydecyl dihydrogen phosphate). This monomer has two functional groups: a divalent phosphoryl group that may adsorb onto zirconia, and a methecryloyl group that can copolymerize with other monomers in the adhesive. This treatment combination has proven to be highly effective.36 Described by Blatz et al, the zirconia bonding protocol has been summarized as the APC concept (A, air-particle abrasion; P, zirconia primer; C, adhesive composite resin).37
Contraindications for single-wing bonded bridges include the following: when the adjacent teeth have diastemas, when the edentulous space is too large as compared to the contralateral tooth being replaced, when the proposed abutment has a short clinical crown, when there is inadequate space for the lingual wing, when there is inadequate enamel on the lingual aspect of the abutment tooth, in a high-function patient, when there is a deep overbite and/or unfavorable occlusion, and, finally, in a long span.38
Clinical Technique for Bonded Bridge
There are a number of requirements to achieve a successful bonded bridge. First, the edentulous site must be approximately the same width as the contralateral tooth. There also must be adequate soft tissue to provide esthetic emergence contours for the ovate pontic. Some clinicians may be concerned that the ovate pontic will not adequately maintain the soft tissue and, over time, the tissue will shrink away from the pontic. However, the soft tissue under an ovate pontic has been shown to be quite stable long-term.39 An example of this is shown in Figure 15.
Additionally, to accomplish a bonded bridge, the abutment tooth must be large enough to provide 30 mm2 of enamel surface and a 3 mm connector height.40 The zirconia wing must be at least 0.7 mm thick to provide the required strength.40 Ideally, this space is created orthodontically to ensure there will be minimal reduction of the lingual enamel. For long-term success, the abutment must be bonded to an enamel substrate.
Next, the pontic must have resistance to facial displacement. This is accomplished by adding a slight extension on the pontic adjacent to an unbonded tooth; this will prevent the pontic from moving facially when loaded from the lingual (Figure 16). There should be no preparation on the unbonded tooth, nor should the extension be bonded so flossing may be easily accomplished. There must also be resistance form on the abutment, which can be best achieved with a vertical groove or box on the lateral surface of the abutment, adjacent to the edentulous site (Figure 17).29 There should be no excursive contacts on the pontic. Lastly, an evidence-based bonding protocol is essential for long-term success.
The primary reason for failure of a bonded zirconia bridge is de-bonding. Porcelain chipping is also possible if layered zirconia is used on the pontic. There is minimal risk of substrate fracture, however, when the aforementioned guidelines are followed.29 If the bridge de-bonds, it can re-prepared for bonding and will not require another time-consuming laboratory procedure. The informed consent should be clear that the bridge will likely de-bond sometime in the future. The patient should be instructed to save the bridge so that it may be re-bonded. The patient should also be given an Essix retainer so that if and when the bridge de-bonds, the retainer will be available to hold the bridge in place for esthetic purposes until it can be re-bonded.
Conclusion
This article has presented treatment options for the replacement of a missing maxillary lateral incisor. A case can be made for the use of any of these treatment options, depending on the clinical situation. However, because of its reasonable cost, good esthetics, and durability, the single-wing bonded zirconia bridge may be considered a primary treatment option.
Acknowledgment
The authors thank Tito Norris, DDS, for the images in Figures 1, 2, and 5 through 10; Jason B. Cope, DDS, PhD, for the images in Figures 11 through 14; and LeBeau Dental Laboratory for the case illustrated in Figures 15 and 16.
About the Authors
J. William Robbins, DDS, MA
Adjunct Clinical Professor, Department of Comprehensive Dentistry, University of Texas Health San Antonio School of Dentistry, San Antonio, Texas; Private Practice, San Antonio, Texas
Marcela G. Alvarez, DDS, MSD
Private Practice, San Antonio, Texas
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Zilberman Y, Cohen B, Becker A. Familial trends in palatal canines, anomalous lateral incisors, and related phenomena. Eur J Orthod. 1990;12
(2):135-139.
2. Polder BJ, Van't Hof MA, Van der Linden FP, Kuijpers-Jagtman AM. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epdemiol. 2004;32(3):217-226.
3. Kokich VO Jr, Kinzer GA. Managing congenitally missing lateral incisors. Part I: canine substitution. J Esthet Restor Dent. 2005;17(1):5-10.
4. Kinzer GA, Kokich VO Jr. Managing congenitally missing lateral incisors. Part II: tooth-supported restorations. J Esthet Restor Dent. 2005;17(2):76-84.
5. Kinzer GA, Kokich VO Jr. Managing congenitally missing lateral incisors. Part III: single-tooth implants. J Esthet Restor Dent. 2005;17(4):202-210.
6. Cope JB, McFadden D. Temporary replacement of missing maxillary lateral incisors with orthodontic miniscrew implants in growing patients: rationale, clinical technique, and long-term results. J Orthod. 2014;41 suppl 1:S62-S74.
7. Norris RT, Caesar RR. Esthetic substitution and autotransplantation of teeth in the maxillary anterior region. Semin Orthod. 2013;19(1):3-12.
8. Garber DA, Salama MA, Salama H. Immediate total tooth replacement. Compend Contin Educ Dent. 2001;22(3):210-218.
9. Cronin RJ Jr, Oesterle LJ. Implant use in growing patients. Treatment planning concerns. Dent Clin North Am. 1998;42(1):1-34.
10. Behrents RG. Growth in the Aging Craniofacial Skeleton. Monograph 17. Craniofacial Growth Series. Ann Arbor, MI: Center for Human Growth and Development, University of Michigan; 1985.
11. Jemt T. Measurements of tooth movements in relation to single-implant restorations during 16 years: a case report. Clin Implant Dent Relat Res. 2005;7(4):200-208.
12. Zachrisson BU. Single implant-supported crowns in the anterior maxilla - potential esthetic long-term (>5 years) problems. World J Orthod. 2006;7(3):306-312.
13. Covani U, Crespi R, Cornelini R, Barone A. Immediate implants supporting single crown restoration: a 4-year prospective study. J Periodontol. 2004;75(7):982-988.
14. Thilander B, Odman J, Jemt T. Single implants in upper incisor region and their relationship to the adjacent teeth. An 8-year follow-up study. Clin Oral Implants Res. 1999;10(5):346-355.
15. Thilander B. Dentoalveolar development in subjects with normal occlusion. A longitudinal study between the ages of 5 and 31 years. Eur J Orthod. 2009;31(2):109-120.
16. Bishara SE, Treder JE, Jakobsen JR. Facial and dental changes in adulthood. Am J Orthod Dentofacial Orthop. 1994;106(2):175-186.
17. Bernard JP, Schatz JP, Christou P, et al. Long-term vertical changes of the anterior maxillary anterior teeth adjacent to single implants in young and mature adults. A retrospective study. J Clin Periodontol. 2004;31(11):1024-1028.
18. Andreasen JO, Paulsen HU, Yu Z, et al. A long-term study of 370 autotransplanted premolars. Part II. Tooth survival and pulp healing subsequent to transplantation. Eur J Orthod. 1990;12(1):14-24.
19. Machado LA, do Nascimento RR, Ferreira DM, et al. Long-term prognosis of tooth autotransplantation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45(5):610-617.
20. Ciarlantini R, Melson B. Semipermanent replacement of missing maxillary lateral incisors by mini-implant retained pontics: a follow-up study. Am J Orthod Dentofacial Orthop. 2017;151(5):989-994.
21. Rochette AL. Attachment of a splint to enamel of lower anterior teeth. J Prosthet Dent. 1973;30(4 Pt 1):418-423.
22. Howe DF, Denehy GE. Anterior fixed partial dentures utilizing the acid-etch technique and a cast metal framework. J Prosthet Dent. 1977;37(1):28-31.
23. Livadtitis GJ, Thompson VP. Etched castings: an improved retentive mechanism for resin-bonded retainers. J Prosthet Dent. 1982;47(1):52-58.
24. Saker S, El-Fallal A, Abo-Madina M, et al. Clinical survival of anterior metal-ceramic and all-ceramic cantilever resin-bonded fixed dental prostheses over a period of 60 months. Int J Prosthodont. 2014;27(5):422-424.
25. Briggs P, Dunne S, Bishop K. The single unit, single retainer, cantilever resin-bonded bridge. Br Dent J. 1996;181(10):371-379.
26. Kern M, Glaser R. Cantilevered all-ceramic, resin-bonded fixed partial dentures: a new treatment modality. J Esthet Dent. 1997;9(5):255-264.
27. Kern M, Passia N, Sasse M, Yazigi C. Ten-year outcome of zirconia ceramic cantilever resin-bonded fixed dental prostheses and the influence of the reasons for missing incisors. J Dent. 2017;65:51-55.
28. Sailer I, Hämmerle CH. Zirconia ceramic single-retainer resin-bonded fixed dental prostheses (RBFDPs) after 4 years of clinical service: a retrospective clinical and volumetric study. Int J Periodontics Restorative Dent. 2014;34(3):333-343.
29. Kern M. RBFDPs. Resin-Bonded Fixed Dental Prostheses. Berlin: Quintessence Publishing; 2018:134-135.
30. Rosentritt M, Preis V, Behr M, Strasser T. Fatigue and wear behavior of zirconia materials. J Mech Behav Biomed Mater. 2020;110:103970.
31. Zhang F, Van Meerbeek B, Vleugels J. Importance of tetragonal phase in high-translucent partially stabilized zirconia for dental restorations. Dent Mater. 2020;36(4):491-500.
32. Klosa K, Meyer G, Kern M. Clinically used adhesive ceramic bonding methods: a survey in 2007, 2011, and in 2015. Clin Oral Investig. 2016;20(7):1691-1698.
33. Özcan M, Bernasconi M. Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent. 2015;17(1):7-26.
34. Usumez A, Hamdemirci N, Koroglu BY, et al. Bond strength of resin cement to zirconia ceramic with different surface treatments. Lasers Med Sci. 2013;28(1):259-266.
35. Blatz MB, Phark JH, Ozer F, et al. In vitro comparative bond strength of contemporary self-adhesive resin cements to zirconium oxide ceramic with and without air-particle abrasion. Clin Oral Investig. 2010;14(2):187-192.
36. Blatz MB, Chiche G, Holst S, Sadan A. Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int. 2007;38(9):745-753.
37. Blatz MB, Alvarez M, Sawyer K, Brindis M. How to bond zirconia: the APC concept. Compend Contin Educ Dent. 2016;37(9):611-617.
38. Kern M. RBFDPs. Resin-Bonded Fixed Dental Prostheses. Berlin: Quintessence Publishing; 2018:28-36.
39. Bienz SP, Sailer, I, Sanz-Martin I, et al. Volumetric changes at pontic sites with or without soft tissue grafting: a controlled clinical study with a 10-year follow-up. J Clin Periodontol. 2017;44(2):178-184.
40. Kern M. RBFDPs. Resin-Bonded Fixed Dental Prostheses. Berlin: Quintessence Publishing; 2018:74-75.